
| ʵ���� | ��Ʒ������/�� | ϡ���������/�� | �������������/�� |
| 1 | 6.0 | 10.0 | 0.88 |
| 2 | 6.0 | 20.0 | 1.76 |
| 3 | 11.0 | 40.0 | 3.52 |
| 4 | 11.0 | 50.0 | X |
| 5 | 11.0 | 60.0 | 4.4 |
���� ��1����1�ݺ͵�2�ݱȽϿ��Է��֣���ϡ�������������ʱ�����������Ҳ�����ӣ�˵����1����̼���û����ȫ��Ӧ��ϡ������ȫ��Ӧ���ݴ˷���X��ֵ��
��2�����ݱ��������ݷ�������ʯ��ʯ��Ʒһ��ʱ��ϡ����Ͳ���������̼��������ϵΪ10g��0.88g���ݴ˽��з����жϣ�
��3���������⣬11gʯ��ʯ��Ʒ��50gϡ������ȫ��Ӧ����������̼������Ϊ4.4g���ݴ˿��Լ�����μӷ�Ӧ��̼��Ƶ�������50g���������ʵ��������������ɵ��Ȼ��Ƶ�������
��4������������ɼ����̼��Ƶ�����������
��5���������з�������ش�
��� ����𡿽⣺��1����1�ݺ͵�2�ݱȽϿ��Է��֣���ϡ�������������ʱ�����������Ҳ�����ӣ�˵����1����̼���û����ȫ��Ӧ��ϡ������ȫ��Ӧ���ɴ˿�֪��ÿ10g��������ȫ��Ӧ���ɶ�����̼������Ϊ0.88g��50g��������ȫ��Ӧ���ɶ�����̼������Ϊ��4.4g������XΪ4.4g��
��2���ɱ��е�����4��5��֪��11g��ʯ��ʯ��Ʒ��50g������ǡ����ȫ��Ӧ��6g��ʯ��ʯ��Ʒ��Ӧ��������Ӧ��y��11g��50g=6g��y ���y��28g����������ʵ���У�ʯ��ʯ��Ʒ��̼���δ��Ӧ�����ı����123��
��3����11gʯ��ʯ��Ʒ��̼��Ƶ�����Ϊm��50g���������ʵ�����������n�����ɵ��Ȼ��Ƶ�����Ϊp
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
m n p 4.4g
$\frac{100}{m}=\frac{73}{n}=\frac{111}{p}=\frac{44}{4.4g}$
��ã�m=10g n=7.3g p=11.1g
ϡ���������ʵ����������ǣ�$\frac{7.3g}{50g}��100%$=14.6%
��4��ʯ��ʯ��Ʒ��̼��Ƶ����������ǣ�$\frac{10g}{11g}��100%$��90.9%
��5��������������֪����������ʵ���У�ʯ��ʯ��Ʒ��ϡ����ǡ����ȫ��һ������5�����鷴Ӧ��������Һ�����ʵ����������ǣ�$\frac{11.1g}{10g+50g-4.4g}��100%$��20%
�ʴ𰸣���1��4.4g����2��1��2��3����3��14.6%����4��90.9%����5��4����Ӧ��������Һ�����ʵ�����������20%��
���� ������Ҫ����ѧ�������ݵķ���Ӧ�����������û�ѧ����ʽ���ۺϼ��㣬����ʹ�����ݡ�������û�ѧ����ʽ�ļ����ۺϷ����ͽ��ʵ����������ȷ��������Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ɱ������� | B�� | ���������������� | ||
| C�� | ��������ˮ������ | D�� | ��ˮ������þ��ʯī |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
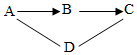 A��D�dz��л�ѧ�������������ʣ�A��B��C�ж���ͬһ��Ԫ�أ�A��C����ʹD�Ӻ�ɫ���ɫ����������ʾ���˵�������ת������-����ʾ�������ʼ�ᷢ����Ӧ�������ʼ��ת����ϵ��ͼ��ʾ���밴Ҫ����գ�
A��D�dz��л�ѧ�������������ʣ�A��B��C�ж���ͬһ��Ԫ�أ�A��C����ʹD�Ӻ�ɫ���ɫ����������ʾ���˵�������ת������-����ʾ�������ʼ�ᷢ����Ӧ�������ʼ��ת����ϵ��ͼ��ʾ���밴Ҫ����գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʳ�︯���������� | |
| B�� | �㡢Ϻ������ˮ�����棬������������������ˮ | |
| C�� | ��ȼ��Ӵ��������ܹ�ȼ�� | |
| D�� | ������������ȼ�գ���������֧���������ʵ�ȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| X | Y | ������̼ | ˮ | ���� | |
| �������� | 78% | 21% | 0.03% | 0.06% | 0.91% |
| �������� | 75% | 15% | 3.68% | 5.44% | 0.88% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��˿�ڿ�����ȼ�գ��������䣬���ɺ�ɫ���� | |
| B�� | ľ̿��������ȼ�գ������⣬���ɶ�����̼ | |
| C�� | �����������ȼ�գ���������ɫ���棬����һ�ִ̼�����ζ������ | |
| D�� | �����ڿ�����ȼ�գ����ɴ����İ�ɫ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com