
����ơ���һ�ֿ�����ϸ�����Ⱥܸߵ�̼��Ʒ�ĩ���й㷺����;��������������Ƭ�����εȡ�����ij��Ƴ��õ��طḻ��ʯ��ʯ��ͨ�����������ơ���ơ���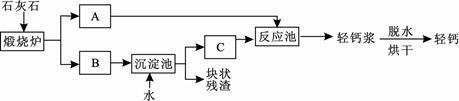
��1��̼����и�Ԫ�ص�����������_________%��ʳ��������̼��������ڷ�ֹ����ȱ
�������_______________________�����������֢����ƶѪ��������
��2��ʯ��ʯ������ת��ΪA��B���÷�Ӧ����_____________���������Ӧ���ͣ���
��3���������еõ��Ŀ�״�������ܺ���δ����ʯ��ʯ��������Ա��������м��飬�۲쵽 ��֤�������к���ʯ��ʯ��
��4������������Ա���������̼���ƴ��������̼���������Ʒ�Ӧ��������̼��Ƶ�ͬʱ���ɵõ���������(һ����Ҫ�ļ����Ӧ��ԭ����Na2CO3+Ca(OH)2=CaCO3��+2NaOH����ͨ���������������ַ�������50t̼���ʱ���ܵõ��������Ƶ������Ƕ��٣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ѧʵ���ᳫ��ɫ��������ʵ��װ�ý����ͻ��Ľ���һ���ܺõ�;����ͼ����ʵ������ȡ������CO2��װ�ã�ͼ���Ƕ�ͼ��ʵ��װ�ý��С��͡����Ľ����װ�ã�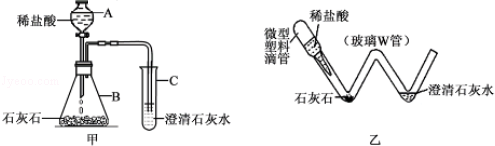
��1��ͼ���������ϵι���ʵ���е�������ͼ���е� ������ͬ������ĸ��ţ���
��2��ͨ���ü�װ����ɸ�ʵ����Ҫ�������ǡ��͡�ʵ��װ��������10�������á��͡�ʵ��װ�þ��е��ŵ��� ��
��3������װ����ɡ���ȡ������CO2����ʵ�飬������0.73��10%�����ᣮ�����ʵ������в���CO2�������Ƕ��ٿˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ȡ���ɿ�����غͶ������̵Ĺ���������Թ��м���������������ų������Թ��ڵ�ʣ�������ȴ������ˮ�����ˡ�ϴ�ӡ�����õ���������3.25g�����õ�100g������������Ϊ7.45%����Һ���Լ��㣨д����Ҫ�ļ�����̣���
��1����Ӧ����������������
��2��ԭ���������Ԫ�ص�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijУ��ѧ��ȤС����һƿ���������Ȼ��Ƶ������ƻ�����Ʒ��������ͼ��ʾ��ʵ�飮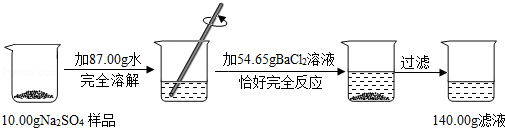
�����������Ϣ���㣨���������С�������λ��
��1����Ӧ���ɳ���������Ϊ�� ��g��
��2����Ʒ�������Ƶ�������
��3������Һ���Ȼ��Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��1����������SO3���У���Ԫ������Ԫ�ص����������� ������Ԫ�ص������������� ����
��2������ij�ȼ���ŷŵ�β���ﺬ�ж���������Cl2����Ϊ��ֹ����Ⱦ������������20%��NaOH��Һ������������Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaClO+NaCl+H2O�������㣺4t������������Ϊ20%��NaOH��Һ�������Ͽ���������������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���к����ʵ��Ȼ�þ��Ʒ10g�����ʲ�����ˮ��Ҳ���μӷ�Ӧ���������м���һ����������������Һǡ����ȫ��Ӧ�����ˣ��õ�117g��������Ϊ10%����Һ����
��1����Ʒ���Ȼ�þ������������
��2������������������Һ��������������������ȷ��0.1%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������������
| A���Ʊ�NaOH��Һ�������͵Ĵ�����Һ��������ʯ��ˮ��Ϻ���� |
| B������̼������ӣ���Ҫ�����������ϡ�����ϣ��۲��Ƿ�������� |
| C������O2��N2��CO2�������壺�ֱ�ȼ�ŵ�ľ�����뼯��ƿ�ڣ��۲����� |
| D����֤�����غ㶨�ɣ�ѡ��CuSO4��Һ��NaOH��Һ���Ƚϻ��ǰ����Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���³�ѹ����ʵ�����ռ�NH3(����)����ѡ���ռ������ص���Ϣ�ǣ� ��
| A��NH3���ܶ� | B��NH3��ˮ�е��ܽ��� | C���������ܶ� | D��NH3������ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com