
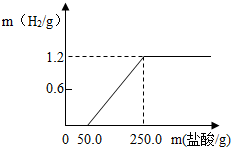
 ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У��������������������������������ͼ��ʾ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�
ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У��������������������������������ͼ��ʾ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�| ���������������� |
| ����������������+��������������� |
| 54 |
| x |
| 219 |
| 200.0g��21.9% |
| 102 |
| y |
| 219 |
| 50.0g��21.9% |
| 27��2 |
| 27��2+16��3 |
| 2.7g |
| 2.7g+10.8g |


 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?ƽ����ģ�⣩ij��ȤС��ӷ������ײ���һ����Ƭ����������20%��ϡ�����У��������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�
��2013?ƽ����ģ�⣩ij��ȤС��ӷ������ײ���һ����Ƭ����������20%��ϡ�����У��������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2011��㶫��25�⣩ij��ȤС��ӷ������ײ���һ����Ƭ����������21.9%��ϡ�����У����������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�
��1����ͼ�п������÷�ӳ������H2_______g��
��2�����������Ļ�ѧ����ʽΪ��__________����Ƭ�������ʵ�����Ϊ____��
��3������������������ռδ����ʱ��Ƭ������������������д��������̡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡƽ����ѧҵˮƽģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�������
ij��ȤС��ӷ������ײ���һ����Ƭ����������20%��ϡ�����У����������������������������������ͼ��������������Ĥ��Ӧʱû��H2�������������ʲ����ᷴӦ������ش�

��1����ͼ�п������÷�ӳ������H2_______g��
��2�����������Ļ�ѧ����ʽΪ��__________����Ƭ�������ʵ�����Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ�麣���п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com