

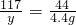
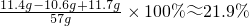
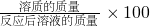 %����Һ��������֪������Ϊ�Ȼ��ƣ�����ԭ������е��Ȼ��ƣ����ݶ�����̼���������̼���Ƶ���������ԭ����������-̼���Ƶ�������Ϊԭ��������Ȼ��Ƶ��������ͷ�Ӧ���ɵ��Ȼ��ƣ����ݶ�����̼�������������
%����Һ��������֪������Ϊ�Ȼ��ƣ�����ԭ������е��Ȼ��ƣ����ݶ�����̼���������̼���Ƶ���������ԭ����������-̼���Ƶ�������Ϊԭ��������Ȼ��Ƶ��������ͷ�Ӧ���ɵ��Ȼ��ƣ����ݶ�����̼�������������


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)ȡֻ���������Ȼ������ʵķ�ĩ״������Ʒ11.4g���ձ��У���μ���ϡ���������ٲ�������Ϊֹ��������ϡ���������Ϊ50g����Ӧ��Ƶ��ձ�����Һ������Ϊ57g(�ٶ���Ӧ�����Ķ�����̼ȫ���ݳ���ˮ�����ӷ����Բ���)��������ش��������⣺
(1)�����漰���Ļ�ѧ��Ӧ����ʽΪ ��
(2)���������غ㶨�ɣ�����������зų�������̼������Ϊ g��
(3)���㷴Ӧ����Һ�����ʵ���������(д����ϸ�ļ�����̣����ݾ�ȷ��l��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ��������ݾ�����ѧ ���ͣ�������
(8��)ȡֻ���������Ȼ������ʵķ�ĩ״������Ʒ11.4g���ձ��У���μ���ϡ���������ٲ�������Ϊֹ��������ϡ���������Ϊ50g����Ӧ��Ƶ��ձ�����Һ������Ϊ57g(�ٶ���Ӧ�����Ķ�����̼ȫ���ݳ���ˮ�����ӷ����Բ���)��������ش��������⣺
(1)�����漰���Ļ�ѧ��Ӧ����ʽΪ ��
(2)���������غ㶨�ɣ�����������зų�������̼������Ϊ g��
(3)���㷴Ӧ����Һ�����ʵ���������(д����ϸ�ļ�����̣����ݾ�ȷ��l��)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com