
��ͼ�Ǽ�ת��Ϊ�����۹��̡�����˵������ȷ����

A. ��Ӧǰ��ԭ������� B. ת�����м�O2��Ӧ�ķ��Ӹ�����Ϊ1��1
C. ת�����ǻ��Ϸ�Ӧ D. �����⡢����Ԫ�ص�������Ϊ1��16��32
B �������� ������ʾ��ͼ��֪��������H2S����������SO2����������H2SO4�� A�����������غ㶨�ɿ�֪����ѧ��Ӧǰ��ԭ�ӵ����ࡢ��Ŀ���������䣬���������⣻B��ת���ٷ����Ļ�ѧ����ʽ��2H2S+3O22SO2+2H2O����O2��Ӧ�ķ��Ӹ�����Ϊ2��3���������⣻C��ת�����Ƕ�������������ⷴӦ�������ᣬ�Ƕ������ʷ�Ӧ����һ�����ʣ����ڻ��Ϸ�Ӧ�����������⣻D�������⡢...
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ�������DZ���С������С�������2018���п���ѧ�Ծ� ���ͣ�������
Сǿ��ȡһ����NaOH�������ձ��У��۲����ڿ����еij�������������һ��ʱ��Ƶù�������Ϊ15.0gȻ������ձ�����μ���һ����������������ϡ���ᣬ��һ���۲쵽�ձ���������ð�������ձ��в���ð������ʱ�����μ�ϡ�����������200.0g��ʱ�Ƶ��ձ�����Һ������Ϊ213.9g����ش�
��1���ձ���ð���������������_____g
��2��Сǿ����NaOH������泱ʪ������ϡ����������ð���������ж�NaOH���峱�Ⲣ�ѱ��ʣ�NaOH���ʵĻ�ѧ����ʽΪ_____������ʺ�Ĺ����к�̼���Ƶ�����_________����д��������̣�
1.1 2NaOH+CO2=Na2CO3+H2O �� 2.65g�� �������� �������Ʊ�������Ϊ�Ϳ����еĶ�����̼��Ӧ����̼���ƺ�ˮ��̼�����ܺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ݷ�Ӧ���ɵĶ�����̼�������̼���Ƶ������� ���������غ㶨�ɿɵã����ɵĶ�����̼������Ϊ15.0g��200.0g?213.9g��1.1g ��μӷ�Ӧ��̼���Ƶ�����Ϊx Na2CO3��2HCl�T2N...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018���п���ѧ�Ծ� ���ͣ���ѡ��
������������ȷ���ǣ�������
A. ����ú��й¶������������������
B. ������������������
C. �ƾ��������ľƾ��Ż���ʪĨ������
D. ����վ����۳��ȵ��Ͻ��̻�
A �������� A��Ϊ��ֹú���Ϳ�����Ϻ�����ը������ú��й©Ӧ�����رշ��Ų�����ͨ�磬�ʴ��� B�������������������ϣ��Է�����©���������֣�����ȷ�� C�����������ϵľƾ�ȼ��������������ʪĨ����ɳ���������ý��º�������������𣬹���ȷ�� D����Ϊ������ڿ�ȼ�Է۳���ȼ���������ڿ�ȼ�����壬��������ϴﵽһ���̶�ʱ������ᷢ����ը��������ۼӹ���������վҪ�Ͻ�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����2018���п���ѧ�Ծ� ���ͣ������
ѡ�������ʵ���������գ�ѡ����ţ���
A���ɱ� B������ϩ C������� D��̼�����
E���ռ� F������̿ G����ʯ�� H��������ϩ
��1�����ڱ������ζ����_________�� ��2������ˮ�¶����ߵ���________��
��3���������˹��������_________�� ��4�����������Ϸ��ϵ���________��
��5���ɸ���������������_________�� ��6�������ڰ�װʳƷ����__________��
F E A C G B �������� �������ʵ������ж����ʵ���;�� ��1������̿���������ԣ���������ζ�����F����2���ռ����������Ƶ��׳ƣ�����ˮ���ȣ��¶Ȼ����ߣ����E����3�����ڸɱ�������ʱ�����մ������ȣ��������˹����꣬���A����4��������к��м�Ԫ���뵪Ԫ�أ����ڸ��Ϸʣ����C����5����ʯ�����������е��ᷴӦ�����ԣ�ũҵ�����г�����ʯ�����к������е��ᣬ���G����...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����2018���п���ѧ�Ծ� ���ͣ���ѡ��
��Cu2(OH)2CO3[Mr=222]��Cu�Ļ����25.4g���ڿ����м���һ��ʱ�䣬��ʣ�����23.1g����ù����м���300.0g9.8%��ϡ���ᣬ������ȫ�ܽ⣬��÷�Ӧ������ʵ������9.8%��ϡ����250.0g������˵����ȷ����
A. ʣ�����Ϊ������
B. �������ȹ����й�����H2O��CO2������Ϊ2.3g
C. ԭ�������Cu2(OH)2CO3��Cu��������Ϊ111��16
D. ����������Һ����Ϊ318.7g
C �������� ʣ������м���ϡ����֮�������ȫ�ܽ⣬˵��ʣ������в�����ͭ���ʡ� ��ʣ�������ͭԪ�ص�����Ϊx ����Cu����H2SO4 64 98 x 250g9.8% x=16g ��ʣ�����������ͭ������Ϊy����ʽ̼��ͭ������Ϊz ����y+z=23.1g�� ���y=12g��z=11.1g ��ԭ������ͭ������Ϊp����ʽ̼��ͭ������Ϊ...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����2018���п���ѧ�Ծ� ���ͣ���ѡ��
���й����ȷ����
ѡ �� | �� �� | ���� |
A | ��ʯȼ�� | ú��ʯ�͡���Ȼ�� |
B | Ӫ������ | ���ࡢ��֬�������� |
C | �����ļ� | ��������ơ��������� |
D | �ϳɲ��� | ���ڡ��л��������ϳ��� |
A. A B. B C. C D. D
C �������� �ɽ��������ӻ�笠����Ӻ���������ӹ��ɵĻ���������Ρ� A��ú��ʯ�ͺ���Ȼ�����ڳ���Ļ�ʯȼ�ϣ�����B��Ӫ��������Ҫ�������ࡢ��֬�������ʡ�ά���ء�ˮ�����Σ�����C��������̼���ƣ������Σ��������ƺ������������ڼ��ȷ��D�������ĺϳɲ��������ϡ��ϳ���ά�ͺϳ��ȣ�����ѡC���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶��һ��������⻯ѧ�Ծ� ���ͣ�ʵ����
ʵ����ѧϰ�����ŷdz���Ҫ�����ã�ijУ��ѧ��ȤС��ѧϰ���������ȡ���ռ������֪ʶ�����ܽᣬ����һ����룬�����������Ŀ���ݣ�
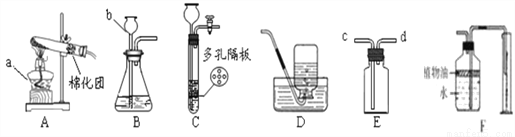
(1)д�������������ƣ� b___________��
(2)��װ��E����ˮ��Ҫ���ų�װ��E��ˮ��ʹ������������������Ӧ��__________��ͨ��(�c����d��)��
(3)ʵ������װ��A��ȡ����ʱ�������Ļ�ѧ��Ӧ����ʽ______________��װ��B��C����������ȡ������̼��װ��C�����װ��B�ڲ���������ŵ���______��
(4)����F�ռ�CO2��Ҫ�������ɵ�CO2����������������ˮ���Ϸ�һ��ֲ����Ŀ����______�� װ��F��������__________��
����©�� d 2KMnO4K2MnO4+MnO2+O2�� �ܹ���ʱ���Ʒ�Ӧ�ķ�����ֹͣ ��ֲ���Ϳ��Է�ֹ������̼����ˮ��Ӱ��������� �������ɵ�CO2�������� �������� ������Ҫ���������������ơ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д�ȣ��ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йء� (1)...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018����꼶����ģ�⿼�ԣ�������ѧ�Ծ� ���ͣ������
Ҫѧ���֤�ؿ�����������������������һ����___________________________��CO������������������һ����_____________________������ЧӦ�����ķ�����_____________��
����ʹʳƷ���ʻ�����ʹ������ʴ������ʹ���ּӾ�� CO���п�ȼ�ԣ�����ȼ�ϻ�CO���л�ԭ�ԣ�������ұ�������� ʹ�������±�������������������¶ȷ�Χ��(��������) �������� �������������ԣ���֧�ֺ�����֧��ȼ�գ������������ʹʳƷ���ʻ�����ʹ������ʴ������ʹ���ּӾ�ȣ�CO���п�ȼ�ԣ�����ȼ�ϻ�CO���л�ԭ�ԣ�������ұ�������ȣ�����ЧӦ��ʹ�������±�������������������¶ȷ�Χ�ڡ� ...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������2018���п���ѧ�Ծ� ���ͣ���ѡ��
�й���Һ��˵����ȷ����( )
A. ��Һ������ɫ�� B. ϡ��Һһ���Dz�������Һ
C. �����ܽ�ʱ���ų����� D. ��Һ���Ǿ�һ���ȶ��Ļ����
D �������� A����Һ��һ������ɫ�ģ�����ͭ��Һ����ɫ�ģ��Ȼ�������Һ��dz��ɫ�ģ�����B��ϡ��Һ��һ���Dz�������Һ��Ҳ�����DZ�����Һ�����������Ƶı�����ҺΪϡ��Һ������C�������ܽ�ʱ��һ�����ų�������Ҳ�������ȣ��������ƹ�������ˮ�ų�������������粒�������ˮ��������������D����Һ�ı��������Ǿ�һ�ԡ��ȶ��ԣ����ڻ�ϣ���ô��Һ���Ǿ�һ���ȶ��Ļ�����ȷ����ѡD���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com