
��ͼװ�ÿ����� CO��ԭFe2O3��ʵ�鲢����÷�Ӧ����������� ��֪��һ����̼����װ�õõ���CO�л�������CO2��H2O��
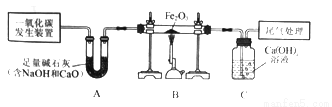
(1)д��Bװ�ò������ڷ�Ӧ�Ļ�ѧ����ʽ___________��
(2)�ӻ����Ƕȿ��ǣ���д��һ��β������������___________
(3)��û��Aװ�ã����ʵ�鲻�ܴﵽ���������������Ŀ�ģ���˵��ԭ��___________
Fe2O3+3CO����2Fe+3CO2 װ��β����һյȼ�ŵľƾ��� CO��ԭ����CO2������֤����ԭ����CO2�������ɵ�CO2ʹCa(OH)2��Һ����� ��������(1)����һ����̼���������ڸ��µ������·�Ӧ�������Ͷ�����̼���(2)���ݽ�β��ȼ�մ������(3)����CO��ԭ����CO2������֤����ԭ����CO2�������ɵ�CO2ʹCa(OH)2��Һ����ǽ��(1)һ����̼���������ڸ��µ�����...
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������ҵˮƽ�߸�����������ģ�⻯ѧ�Ծ� ���ͣ�������
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ�����ᷢչ����������˾��ס��ϳɰ��Ĺ�������ͼ���£�
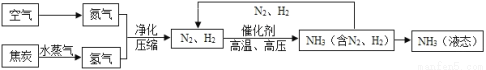
(1)���������ɵİ�(NH3)����ũҵ���Ǻϳ�_________(����ʡ����ʡ��طʡ�)��ԭ�ϡ�
(2)��ȡ�����ķ�Ӧ����Ϊ��C + H2O CO + H2�����з�����ԭ��Ӧ��������_____________��
CO + H2�����з�����ԭ��Ӧ��������_____________��
(3)д��N2��H2��Ӧ����NH3�Ļ�ѧ����ʽ_________________________________��
(4)�������п�ѭ��ʹ�õ�������___________________��
(5)���е㲻ͬ��������뿪����������Һ�����뷨���±��Ǹ����ʵķе㡣
���� | H2 | N2 | O2 | NH3 |
�е� | �C 252�� | �C 195.8�� | �C 183�� | �C 33.35�� |
������¶�t��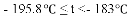
A��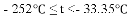
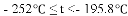
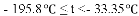
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ������2018����꼶6���¿���ѧ�Ծ� ���ͣ���ѡ��
�����д����л�ѧ����ʵ�����������ʵ�������;���仯ѧ�����ص��� ( )
A. �û���̿�����ж����� B. ��ľ̿�����
C. ��ϡ�������ˮƿ�е�ˮ�� D. ��������ʯ��ʯ����������̼
A ��������A������̿�����������������ʣ�����������û�����������ɣ����뻯ѧ�����أ�����ȷ�� B����ľ̿�����������̼��ȼ�գ��뻯ѧ�����йأ��ʴ��� C����ϡ�������ˮƿ�е�ˮ������ϡ������̼��Ʒ�Ӧ�Ĺ��̣��ù����������������ɣ����ǻ�ѧ�仯���뻯ѧ�����йأ��ʴ��� D����������ʯ��ʯ����������̼�ǻ�ѧ�仯�����뻯ѧ�����йأ��ʴ���ѡA���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ��������2018���п���ѧ�Ծ� ���ͣ���ѡ��
��������ʵ���۽�����ȷ����
ѡ�� | ��ʵ | ���� |
A | ���ʯ��Ӳ��Զ����ʯī | ̼ԭ�ӽṹ��ͬ |
B | ������Һ���ܵ��� | ��Һ��û�������ƶ������� |
C | 6000L�����ڼ�ѹ������¿�װ���ݻ�Ϊ40L��ƿ�� | �����ӱ�С�� |
D | ϡ���ᡢϡ����������� | ��Һ�ж����������� |
A. A B. B C. C D. D
D ��������A�����ʯ��Ӳ��Զ����ʯī������Ϊ̼ԭ�����з�ʽ��ͬ������B��������Һ���ܵ��磬����Ϊ��Һ��û���������ƶ��Ĵ��е�ɵ����ӣ�����C��6000L�����ڼ�ѹ������¿�װ���ݻ�Ϊ40L��ƿ�У�����Ϊ�����Ӽ����С�ˣ�����D��ϡ���ᡢϡ����������ԣ�����Ϊ��Һ�ж����������ӣ���ȷ����ѡD���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ʡ��������2018���п���ѧ�Ծ� ���ͣ���ѡ��
�������ʵ���;�������
|
|
|
|
A.�þ�����ϩ��������������ľ�Ե�� | B.����ʯ�Ҹ����������� | C.���Ȼ�������������ˮ | D.ϡ���������ڴ������г� |
A. A B. B C. C D. D
D ��������A��������ϩ���������õľ�Ե�ԣ�����������ľ�Ե�㣬��ȷ��B������ʯ�Ҹ���������������ȷ��C��������ˮ��0.9%���Ȼ�����Һ����ȷ��D���������ڴ������г�������ѡD���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018���п���ѧ�Ծ� ���ͣ��ƶ���
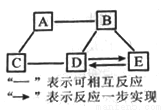
A��E��Ϊ���л�ѧ���������ʣ�����֮��Ĺ�ϵ��ͼ��ʾ(���������Ѿ���ȥ)����֪A��Ŀǰ�������������ߵĽ���;B��θ�����Ҫ�ɷ�;C�н���Ԫ�ص���������Ϊ40%�� ��ˮ��Һ����ɫ������������ũҩ������Һ;D���ڼ�;E�����Ρ���C�Ļ�ѧʽΪ__________;A��B��Ӧ�Ļ�ѧ����ʽΪ__________��Eת��ΪD�Ļ�ѧ����ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018���п���ѧ�Ծ� ���ͣ���ѡ��
��������(Na2O2)���������������������Դ�����������̼��Ӧ���������Ϊ( )
A. Na2CO3��H2 B. Na2O��O2 C. NaOH��O2 D. Na2CO3��O2
D ����������������Ϣ�������غ㶨�ɿ�֪�����������������̼��Ӧ������������һ�����ʣ���һ�������бض�������Ԫ�غ�̼Ԫ�أ���ѡD���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������2018���п���ѧ�Ծ� ���ͣ������
���û�ѧ������ա�
(1)ij���Ľṹʾ��ͼΪ �������ķ���_______________��
�������ķ���_______________��
(2)ϡ�����庤�Ļ�ѧʽ______________��
(3)����˫��ˮ��ѧ���ʵ���С��_______________��
(4)д���������Ļ�ѧʽ�������Ԫ�صĻ��ϼ�_______________��
Cl- He H2O2 ����������1�������ӽṹʾ��ͼ�к���������Ⱥ�����������1�����������ӣ�����Ϊ Cl- �� ��2��ϡ��������ԭ�ӹ��ɣ��仯ѧʽ��ԭ�ӷ��ű�ʾ�����Ļ�ѧʽ��He�� ��3�������DZ������ʻ�ѧ���ʵ���С�����ʱ���˫��ˮ��ѧ���ʵ���С����H2O2�� ��4����������������Ļ��ϼ�Ϊ-2�ۣ����ݻ��ϼ۵Ĵ�����Ϊ0�����Ԫ�صĻ��ϼ�Ϊ+3�ۣ���ʾΪ�� ...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2018����꼶��ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ��ۺ���
ʵ����ѧϰ��ѧ����Ҫ������
��ʵ��һ����������ʵ�顱��ͼ��
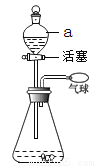
��1��������ͨ������a����ƿ�м�ˮ���۲쵽�����ʹرջ�����һ��ʱ���������Сû�б仯��˵����װ��������_______��
��2������������ʹ�����ʹ���Ӧ�Ļ�ѧ����ʽΪ_______��
��3������ƿ��װ���������ƹ��壬ͨ������a������ˮ������Ҳ���ʹ���Ҫԭ����____��
��ʵ�����̽����ͼ������
��1�����ˮʱ������NaOH��ǿˮ�ĵ����ԣ�������NaOH��ˮ���ܽ����______��д���ӷ��ţ���
��2����ͼΪϡ����������������Һ��Ӧ��������ҺpH�����Һ������仯���ߡ�
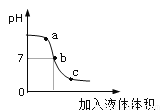
�ٲⶨ��ҺpH�ķ����ǣ��ò�����պȡ��Һ���ε�pH��ֽ�ϣ�����ֽ��ʾ����ɫ��_____���գ���ȡpH��ͼ2��a���Ӧ����Һ��______�ԣ�������Һ�м���____����Һ��Ϊ��ɫ��Ҳ�ܵó��˽��ۡ�
�������ϵ�b��˵������Һǡ����ȫ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ_______��
��3����ͼ����ע��������Һ���CO2(����Ⱦ�Ϊ1�U5)��Ȼ��н����ɼУ�������
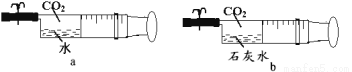
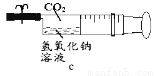
������______���a��b��c��������ĶԱȣ�����Ϊ�������ƺͶ�����̼������Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ______��
����Ҫ֤��a��Ҳ�����˻�ѧ��Ӧ�����ʵ�鷽���ǣ�д������������______��
���� 2H2O22H2O +O2�� �������ƹ����ܽ�ʱ���� Na+��OH�� ����ɫ�� �� ��̪ 2NaOH+H2SO4=Na2SO4+2H2O ac 2NaOH+CO2=Na2CO3+H2O ȡ����a��Һ��μ�ʯ����Һ�����ɫ �����������⿼���˼��װ�õ������ԣ�ʵ������ȡ�����ķ�Ӧԭ�����ܽ�ʱ�����Ȼ����������ͼ�����ʡ��ѶȲ��������е�֪ʶ���з������ ʵ��һ����1����...�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com