
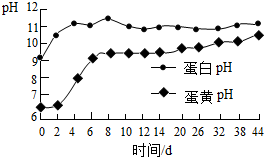
���� ��1�����ݻ���������������������ʼ������ʣ������кͷ�Ӧ�����ۣ�ѡ�����ʽ����кͷ�Ӧ���ƿ�ζ���
��2�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص�������𣻸��ݷ�Ӧԭ���������
��3������ͼ����Ϣ�������
��4��A�������ɻ���������ɫ���γ������������йؽ��
B�������ɻ������кܸߵ�Ӫ����ֵ��Ӧ����ʹ�ý��
C�����������ɻ������ܺ���������Ǧ����ͯ����ʳ�ý��
D�����ݰ���Ƥ���ɻ�����ҹ���ú���ܱ��ʣ�����ʳ�ý��
��� �⣺��1���ɻ��������������м����ˡ���ˮ�����ʼ��Ե����ʣ����Դ�ɬζ��Ϊ����ɬζ���ڳ��ɻ���ʱ�����պ�������Ե����ʣ����������кͼ�ˮ����ʳ�������ԣ�����ڳԵ�ʱ��պ��ʳ���ƿ�ζ��
��2���������������л�����ǿ��NaOH��KOH����Ԫ��������ԭ���еIJ�ľ�ң���K2CO3������ʯ������ˮ��Ӧ�����������ƣ��˷�ӦΪ���Ϸ�Ӧ�����ɵ�������������̼���Ʒ�Ӧ���ɲ�����ˮ��̼��ƺ��������ƣ����ڸ��ֽⷴӦ����Ӧ�Ļ�ѧ����ʽΪ��Ca��OH��2+Na2CO3�TCaCO3��+2NaOH��
��3������ͼ����Ϣ��֪�������ɻ�������ʱ�������е���͵���pH�ı仯��ϵ�жϣ�������͵���pH���ﵽ9����ʱ���ɻ����������Ƶ�����Ϊ12�죻
��4��A���ɻ���������ɫ���γ������������йأ�����ȷ��
B���ɻ������кܸߵ�Ӫ����ֵ��Ӧ����ʹ�ã��ʴ���
C�������ɻ������ܺ���������Ǧ����ͯ����ʳ�ã�����ȷ��
D������Ƥ���ɻ�����ҹ���ú���ܱ��ʣ�����ʳ�ã��ʴ���
�𰸣�
��1����ɬ��
��2����ľ�ң���K2CO3���� Ca��OH��2+Na2CO3�TCaCO3��+2NaOH
��3��B��
��4��AC��
���� ������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ�����������ʱҪ��ѧ��������������֮�������ʱ�ķ�Ӧ�����


 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �٢ܢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A����ѧ������ | B����ѧ�뻷�� |
| �������յķ����ܼ��������ߺ���ë�� �ڻ���̿�ܾ�ˮ����Ϊ����̿�������� | ��������Ҫ����CO2��SO2��������� ��PM2.5��������������ġ�Ԫ�ס�֮һ |
| C����ѧ�뽡�� | D����ѧ�밲ȫ |
| �ٷ�ù�Ĺ�����ɹ��ʳ�� ��ʹ�ü������Ϳ�Ԥ��ƶѪ | �پƾ��������ϲ���ȼ��������������ˮ���� ������ؽ�������Һ���ȼ������ţ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �������ʾ����ڻ����� | B�� | ���ɱ��Ͷ���������Ϊ11��7 | ||
| C�� | ���ҵķ��Ӹ�����Ϊ1��2 | D�� | �����ӵ���ʾ��ͼΪ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������̼��Ӧ | B�� | �������ᷴӦ | ||
| C�� | ������������Ӧ | D�� | ��ʹ��̪��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
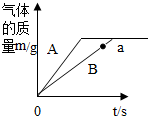 A��BΪ̼��ƿ�״�����̼���Ʒ�ĩ�е�ijһ�֣������ձ��зֱ��������Ϊ16g��A��B�������ʣ�������109.5g��������������ͬ��ϡ���ᣬ��ַ�Ӧ����Ӧ��������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ��
A��BΪ̼��ƿ�״�����̼���Ʒ�ĩ�е�ijһ�֣������ձ��зֱ��������Ϊ16g��A��B�������ʣ�������109.5g��������������ͬ��ϡ���ᣬ��ַ�Ӧ����Ӧ��������������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
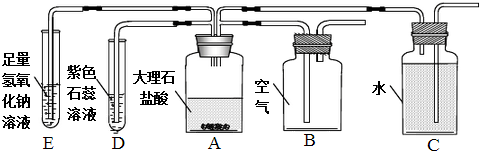
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ǻ��Ϸ�Ӧ | B�� | ���ǷֽⷴӦ | C�� | �����û���Ӧ | D�� | ���Ǹ��ֽⷴӦ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com