С��ͬѧΪ�˲ⶨ����С�մ��θҩ��С�մ�ĺ���������θҩ�������ɷֲ�����ˮ��Ҳ�����ᷴӦ��ȡ20g��θҩ���ձ��У������м���100gϡ���ᣬǡ����ȫ��Ӧ���ձ���ʣ�����ʵ�����Ϊ111.2g�������Ҫ��ش����⣺
��1��������Ӧ�Ļ�ѧ����ʽ______
��2��������֪�����г�ϡ����������������x���ı���ʽ______
��3����θҩ��С�մ�ĺ���Ϊ______
��4����С����ȡһ�ֺ�С�մ��θҩ�������ɷֲ�����ˮҲ�����ᷴӦ�������ƵIJⶨ��ͬ��ȡ20gҩƷ��100g��һ��δ֪Ũ�ȵ����ᣬǡ����ȫ��Ӧ�����ձ���ʣ����ҺΪ106g�������������������������Ϊ______ ��ȡ������
���𰸡�
��������1������С�մ�����ʣ�д��С�մ������ᷢ����Ӧ�Ļ�ѧ����ʽ��
��2�����������غ㶨�ɣ������ǡ����ȫ��Ӧ���ų�������̼�����������ö�����̼�������ڷ�Ӧ�е�������ϵ���г�ϡ����������������x���ı���ʽ��
��3����θҩ��С�մ�ĺ���=

×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼�������������
��4�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɼ���̼�����Ƶ�������ų�������̼��������ϵ���ж���ȫ��Ӧʱ��Һ�����IJ���������Һ������������ķ�Ӧ��ϵ�����òⶨʵ������Һǰ�����������������ϡ���������������
����⣺��1��̼�����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪNaHCO
3+HCl�TNaCl+H
2O+CO
2����
��2�����������غ㶨�ɣ���Ӧ�ų�������̼������=20g+100g-111.2g=8.8g
NaHCO
3+HCl�TNaCl+H
2O+CO
2��
84 36.5 44
x 8.8g
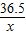
=
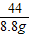
��3����С�մ������Ϊy
NaHCO
3+HCl�TNaCl+H
2O+CO
2��
84 44
y 8.8g
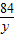
=
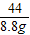
y=16.8g
��θҩ��С�մ�ĺ���=
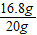
×100%=84%
��4�����������غ㶨�ɣ���Ӧ�ų�������̼����Һ��������=106g-100g=6g
�����������������������Ϊz
NaHCO
3+HCl�TNaCl+H
2O+CO
2�� ��Һ����
84 36.5 44 84-44=40
100g×z 6g
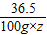
=
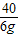
z=5%
�ʴ�Ϊ��
��1��NaHCO
3+HCl�TNaCl+H
2O+CO
2����
��2��
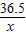
=
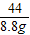
��
��3��84%��
��4��5%��
������������Һ�IJ�����������⣺���ݷ�Ӧ�Ļ�ѧ����ʽ�����÷�Ӧǰ��������Һ����������㷴Ӧ�����������������������������ʹ������̼������࣮
��Ŀ�����л�ѧ
��Դ��2011�������ʡ�������е������п���ѧһģ�Ծ��������棩
���ͣ������
С��ͬѧΪ�˲ⶨ����С�մ��θҩ��С�մ�ĺ���������θҩ�������ɷֲ�����ˮ��Ҳ�����ᷴӦ��ȡ20g��θҩ���ձ��У������м���100gϡ���ᣬǡ����ȫ��Ӧ���ձ���ʣ�����ʵ�����Ϊ111.2g�������Ҫ��ش����⣺
��1��������Ӧ�Ļ�ѧ����ʽ______
��2��������֪�����г�ϡ����������������x���ı���ʽ______
��3����θҩ��С�մ�ĺ���Ϊ______
��4����С����ȡһ�ֺ�С�մ��θҩ�������ɷֲ�����ˮҲ�����ᷴӦ�������ƵIJⶨ��ͬ��ȡ20gҩƷ��100g��һ��δ֪Ũ�ȵ����ᣬǡ����ȫ��Ӧ�����ձ���ʣ����ҺΪ106g�������������������������Ϊ______ ��ȡ������
�鿴�𰸺ͽ���>>

 ×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼�������������
×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼�������������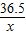 =
=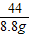
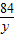 =
=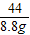 y=16.8g
y=16.8g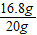 ×100%=84%
×100%=84%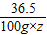 =
=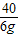 z=5%
z=5%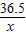 =
=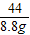 ��
��

 ����������ϵ�д�
����������ϵ�д�