


 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ������

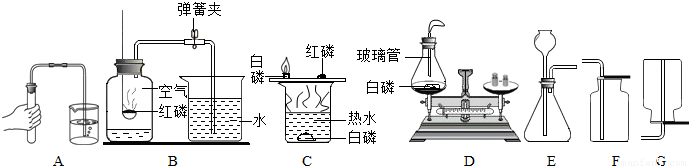
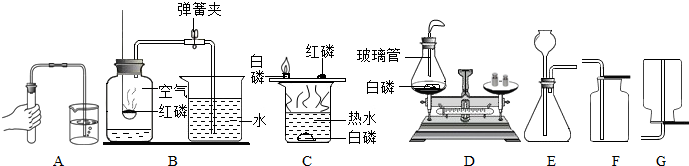
 ��ʵ�飬��ʵ��Ŀ�ķֱ��ǣ��磺B-�ⶨ�����������ĺ�����������C��D����ѡһ������ ��
��ʵ�飬��ʵ��Ŀ�ķֱ��ǣ��磺B-�ⶨ�����������ĺ�����������C��D����ѡһ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
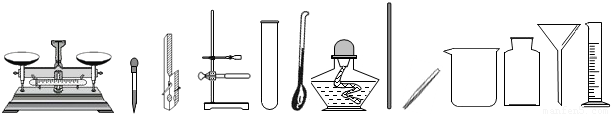
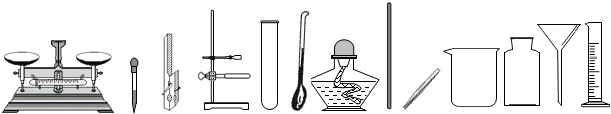
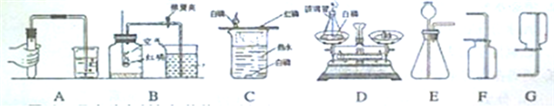
��������ͼʾ�dz��л�ѧ�γ��г��õ�������ʵ��װ�ã��밴Ҫ��ش��-���е����⣺
 �����ڶ��������ģ���дһ���������ƣ��磺������ƽ�� �ȣ�
�����ڶ��������ģ���дһ���������ƣ��磺������ƽ�� �ȣ�
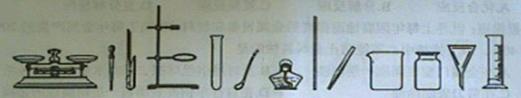
������ȡ��ҩƷ����������дһ���������ƣ��磺���ӡ� �ȣ�
��������������������дһ���������ƣ��磺�Թܡ� �ȣ�
�����ڹ��˵������ǣ���д������Ҫ���������ƣ� ��
 �� ͼʾA��ʵ�����Ʊ�����Ǯ�����IJ��裬�����ֱ���Ϊ ��
�� ͼʾA��ʵ�����Ʊ�����Ǯ�����IJ��裬�����ֱ���Ϊ ��
��B��C��D����������ȼ���йص�ʵ�飬��ʵ��Ŀ�ķֱ��ǣ��磺B—�ⶨ�����������ĺ�����������C��D����ѡһ������ ��
����װ��E����ȡ�������壬����E+F��Ͽ���ȡ�������ǣ�д��ѧʽ�� ������E+G��Ͽ���ȡ����Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com