
ʵ������ȡ�����ǻ�ѧѧϰ�Ļ���ʵ�鼼��֮һ���������ͼ�ش��������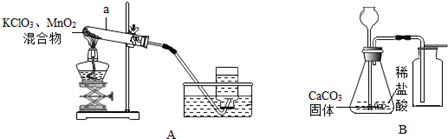
��1�������a�������� ����
��2����ͬѧ��Aװ����ȡ�������÷�Ӧ�Ļ�ѧ����ʽ�� �����÷�Ӧ�Ļ�����Ӧ�������� ����ֹͣ����ʱӦ�Ƚ������Ƴ�ˮ�棬���������� ����
��3�����鼯��ƿ�е������������ķ���Ϊ��
��4����ͬѧ��Bװ����ȡ������̼���÷�Ӧ�Ļ�ѧ����ʽΪ�� �����ռ����Ķ�����̼�п��ܺ��е��������� ����Ҫ��ȥ��Щ�����õ����Լ��Ⱥ����� ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�ʵ����ѡ����ͼ��ʾװ����ȡ���ռ����壬����Ҫ��ش��������⡣
��1��ʵ������Bװ����ȡ����ʱ���йصĻ�ѧ��Ӧ����ʽ�� ��
װ��B����������ȡ�������� ��
��2��ʵ���ҳ�����ˮ�����ƺͼ�ʯ�ҵĻ�Ϲ����ڼ�����������ȡ�������壬����װ�ÿ�ѡ�� ������ţ�����������һ�����װ��C�����ռ����ռ�������ƿ�ķ��÷�ʽ��ͼ��ʾ���ݴ˿��ƶϼ���һ���߱������������ǣ� �� ��
��3����װ��C�ռ�����ǰ��������ƿ�ڵĿ����ž��IJ��������ǣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(8��)ijѧϰС��Χ�ơ������ʵ������ȡ���������֣�������������������⡣
��1��ͼ��װ����ȡ��������������� ��
��2��ͼ��װ�����ͼ��װ�õ���Ҫ�ŵ��� ��
��3��ʵ���Ҳ�����þ������п��ϡH2SO4��Ӧ��ȡH2����Ҫԭ����
��
��4��ʵ��������ͼ��װ���ռ�NH3ʱ������Ӧ�� ��ͨ�롣(����ĸ)
��5��Ϊ�˲ⶨͭԪ�ص����ԭ������Ar(Cu)��ijͬѧ��������ԭ����ͭ�ķ�Ӧ����ˣ�
��ͼ�е�һ��װ�м�ʯ�ҵ�U�ιܵ������� ��
����÷�Ӧǰ����ͭ������Ϊag��ʵ����ɺ�ڶ���װ�м�ʯ�ҵ�
U�ι����ӵ�����Ϊbg����Ar(Cu)= ��(��a��b��ʾ)��
��6�������õ����������������ܡ���Ƥ�ܡ��Թܡ�����ƿ�Ͳ�
��Ƭ�����һ����ȡCO2��ʵ��װ�ã�������ʵ��װ�õļ�ͼ����
����ķ����ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʵ����������װ�ý����������ȡ������ʵ�飮
��1��ѡ��װ��A��ȡ������̼�Ļ�ѧ����ʽΪ�� �����������ſ������ռ������ݶ�����̼�� ����
��2��ѡ��װ��B�ø��������ȡ�����Ļ�ѧ����ʽΪ�� ������ �������ռ�����������������Ϊ�������� ����
��3����װ��C��ϸ��˿��������ȼ��ʵ�飬����ƿ�ײ�Ԥ�ȷ�������ˮ��Ŀ������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4.5�֣���ͼ��ʵ���ҳ��õ�������װ�ã���ͼ�ش��������⡣ 
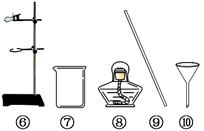
��1��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ ����ѡ�õķ���װ���� ����������ţ���ͬ����ʵ���Ҽ��������̼�Ļ�ѧ����ʽΪ ��
��2��ʵ����������غ������������̵Ļ������ȡ�����Ļ�ѧ����ʽΪ ���ռ�װ��Ϊ �����ӷ�Ӧ��Ļ�����л��ն������̣���Ҫ�õ����˲���������ʱӦѡ�õ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ��ʾ��ʵ��װ�ã���ش����⣺
��1���ڼ���ҩƷ֮ǰӦ��ʵ��װ�ý��� ��
��2���ø��������������ѡ�õķ���װ���� ������ĸ��ţ���ͬ�����ù���������������ѡ�õķ���װ���� ������Ϊ����װ�ò�һ������Ҫԭ���� ��
��3��ijͬѧ��Eװ���ռ������������� ��Ϊʲô���ܿڸ�������ð��ʱ�����������ռ� ��
��4����ѡ��Bװ���Ʊ�����ʱӦע��ʲô ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(4��)ʵ���ҳ��ø�����غ���ͼ��ʾװ����ȡ��������ش��������⣺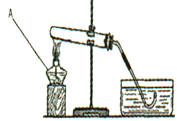
��1����ͼ��װ����ȱ���ֲ���������
��2��д����Ӧ�Ļ�ѧ����ʽ___________________________��
��3���ڴ�ʵ����ʹ������A���Թܼ��ȵ�ע��������___________________________��
��4������ˮ���ռ���һƿ����������˿ȼ��ʵ�飬���Ƴ�����״�Ĺ�����˿�¶�ϵ�Ļ���ȼ��IJ�����___________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ڳ��е�ѧϰ������������������������̼��ʵ������ȡ������ijУ��ѧ��ȤС���ͬѧ�����淶�IJ���Ҫ�����ú�����װ����ʵ������ȡ����Щ���塣��ͼ������ʵ�����ù�����������ش��������⣺
��1��д��ͼ���������������ƣ���_____ _ ��
��2��С��ͬѧ����ȡ����ʱʹ�õ������Ǣ٢ۢܢݢߢ⣬����ȡ������ķ�Ӧ��ѧ����ʽΪ �������ȡ�����巢��װ�õ������Եķ����� ��
���������ķ����� ������ʵ���г����Թ����ѵ�������ԭ������� ����һ�����ɣ���
��3��С��ͬѧ˳������ȡ���ռ���һƿ������̼����֪��ʹ���������ڢޣ���ô������ʹ����ͼ�����е�_______________(�����)����������弯���ķ����������� �����������зſ�״����ҩƷ�ķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ͼ�ش����⣺
��1��ʵ������Bװ����ȡ��������Ӧ�ķ���ʽΪ���� �����ռ�װ����ѡE�����ſ������ռ�������������Ӧ���������a����b���� �˽��룻����װ��F�ռ��������ռ�����ǰ����Ҫ������ƿ���ȳ���ˮ��Ӧ��ν��в�����
��2��ʵ���ҳ���Cװ����ȡCO2����Ӧ��Ҫ�õ��Ȼ��ƹ�����Ҫ�õ����������� װ�ã�����ţ���
��3��ij��ʵ��װ������ͼG����װ���൱����ͼװ�õ�����������ţ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com