
 ������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��
������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��| 36.5 |
| 40 |
| X |
| 8g |
| 7.3g |
| 25g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ�ع����о��꼶������ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ�������
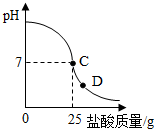
��8�֣�Ϊ�ⶨ������������Ϊ32%�������ʵ������������С��ʵ��ʱ�����ձ��м���20g40%������������Һ������μ�������ᣬ�ⶨ������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��
��1����������������Һ�����ʵ�����Ϊ____________g��
��2�������濴����ͼ��Ϣ��ش���������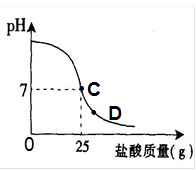
�ٵ��μ����ᵽC��ʱ�������ĵ����������ʵ������Ƕ��٣�
�ڸ������ʵ�����������Ƕ��٣�(������0.1%)
�۵���������Һ�������������ı��ԭ���ǣ� ��
�ܵ��μ����ᵽͼ����D��ʱ���ձ�����Һ�������� �����ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ���꼶������ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�������
��8�֣�Ϊ�ⶨ������������Ϊ32%�������ʵ������������С��ʵ��ʱ�����ձ��м���20g40%������������Һ������μ�������ᣬ�ⶨ������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��
��1�� ��������������Һ�����ʵ�����Ϊ____________g��
��2�� �����濴����ͼ��Ϣ��ش���������
�ٵ��μ����ᵽC��ʱ�������ĵ����������ʵ������Ƕ��٣�
�ڸ������ʵ�����������Ƕ��٣�(������0.1%)
�۵���������Һ�������������ı��ԭ���ǣ� ��
�ܵ��μ����ᵽͼ����D��ʱ���ձ�����Һ�������� �����ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
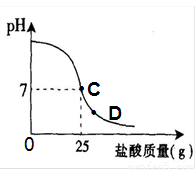
Ϊ�ⶨ������������Ϊ32%�������ʵ������������С��ʵ��ʱ�����ձ��м���20g40%������������Һ������μ�������ᣬ�ⶨ������������������ձ�����ҺpH�Ĺ�ϵ��ͼ��
��1����������������Һ�����ʵ�����Ϊ____________g��
��2�������濴����ͼ��Ϣ��ش���������
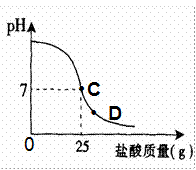
�ٵ��μ����ᵽC��ʱ�������ĵ����������ʵ������Ƕ��٣�
�ڸ������ʵ�����������Ƕ��٣�(������0.1%)
�۵���������Һ�������������ı��ԭ���ǣ� ��
�ܵ��μ����ᵽͼ����D��ʱ���ձ�����Һ�������� �����ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011��㶫ʡ�ع������п���ѧ��ģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com