
���� ��1�����ݹ���������ˮ��Ӧ����ʽ����������������������ƺ�ˮ��������
��2�����ݹ������Ƶ�������������ϵ��������������������ͼ��
��� �⣺��1����������Ƶ�����Ϊx��ˮ������Ϊy��
2CaO2+2H2O=2Ca��OH��2+O2��
144 36 32
x y 32g
$\frac{144}{x}\frac{36}{y}=\frac{32}{32g}$
��ã�x=144g y=36g
���144g��
��2�������������֪��ˮ��������36g�����ɵ�������32g�����Բ�������������ͼ���£�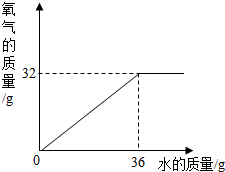
���� ������Ҫ�����˸��ݻ�ѧ����ʽ�ļ��㣬ע����������Ҫȷ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ռNa2CO3 | B�� | ������CuSO4 | C�� | ��ʯ�ң�CaCO3 | D�� | ���ף�P |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ȼ���ˮ���ǻ���� | |
| B�� | ���ˡ����������������ȶ��dz��õľ�ˮ���� | |
| C�� | ˮ��Ca2+��Mg2+���ർ��ˮ�帻Ӫ���� | |
| D�� | �����ϵ�ˮ�����Ƿḻ�ģ����ɹ����õĵ�ˮ��Դ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��̼���⡢������Ԫ����� | B�� | ����������Ԫ�صĺ������ | ||
| C�� | ̼���⡢������Ԫ�ظ�����Ϊ5��5��1 | D�� | ÿ��������2����ԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com