
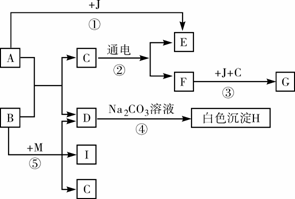
��ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ�(Fe2O3��xH2O)��E��F��IΪ��ɫ���壮����ͼʾ�ش��������⣮

(1)д���й����ʵĻ�ѧʽ��B��________��C��________��
(2)д����Ӧ�ܵĻ�ѧ����ʽ��________��
(3)��Ӧ������________��Ӧ(�Ӧ����)��
(4)�ճ�������Ϊ����ֹ��Ӧ�۷�����ͨ����ȡ�Ĵ�ʩ��________(дһ��)��
(5)Ҫʹ��Ӧ���ܹ���������Ҫ��Ӧ��B��M������M�Ļ�ѧʽΪ________��
 ȫ�ܲ��һ���þ�ϵ�д�
ȫ�ܲ��һ���þ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 26����ͼ��A������θҺ�к��е����ʣ�B�����ڸ�������������J������Ľ�����G�Ǻ���ɫ��ĩ��E��F��IΪ��ɫ���壬I��ʹʪ��ĺ�ɫʯ����ֽ������
26����ͼ��A������θҺ�к��е����ʣ�B�����ڸ�������������J������Ľ�����G�Ǻ���ɫ��ĩ��E��F��IΪ��ɫ���壬I��ʹʪ��ĺ�ɫʯ����ֽ�������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?տ������ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ֣�Fe2O3?xH2O����E��F��IΪ��ɫ���壮����ͼʾ�ش��������⣮
��2011?տ������ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ֣�Fe2O3?xH2O����E��F��IΪ��ɫ���壮����ͼʾ�ش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ���о��꼶5���п�ģ�⿼�Ի�ѧ�Ծ����������� ���ͣ��ƶ���
(6��)��ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ֣�Fe2O3��XH2O����E��F��IΪ��ɫ���塣����ͼʾ�ش��������⡣
��1��д���й����ʵĻ�ѧʽ��B��______��C��______��
��2��д����Ӧ�ܵĻ�ѧ��Ӧ����ʽ��________��
��3����Ӧ������__________��Ӧ���Ӧ���ͣ���
��4���ճ�������Ϊ����ֹ��Ӧ�۷�����ͨ�����õĴ�ʩ��____________��дһ������
��5��Ҫʹ��Ӧ���ܹ���������Ҫ��Ӧ��B��M������M�Ļ�ѧʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
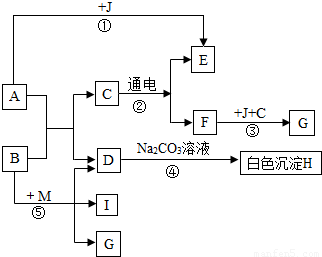
��ͼ��A������θҺ�к��еijɷ֣�B���ڸ�������������G���������Ҫ�ɷ֣�Fe2O3��xH2O����E��F��IΪ��ɫ���塣����ͼʾ�ش��������⡣
��1��д���й����ʵĻ�ѧʽ��B :������������ ��C:������������ ��
��2��д����![]() Ӧ�ܵĻ�ѧ����ʽ:����������������������������������������������
Ӧ�ܵĻ�ѧ����ʽ:����������������������������������������������
��3����Ӧ������ ������������������Ӧ���Ӧ���ͣ���
��4���ճ�������Ϊ����ֹ��Ӧ�۷�����ͨ����ȡ�Ĵ�ʩ������������������ ��дһ������
��5��Ҫʹ��Ӧ���ܹ���������Ҫ��Ӧ��B��M������M�Ļ�ѧʽΪ���������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ������������ѧ���꼶���ϣ���ĩ��ѧ��ϰ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com