���������ͷ�չ�벻����Դ����Դ��
��1���Ͼ�����ȼ�ϵĸ��¹������£�

�������йؼ���ȼ�ϸ��µ����ɣ���ȷ����
BD
BD
��ѡ����ĸ����
A����Ȼ�����ڿ�������Դ
B������ȼ�ϱȹ���ȼ�������ʸ���
C����Ȼ����Ϊȼ�Ͽɱ�������ЧӦ�ķ���
D��ú��������ȼ���յ��˷���Դ
����֪��ͬ�¡�ͬѹ�£���������ȵ��ڷ��Ӹ����ȣ��ܵ�ú������Ҫ�ɷ���һ����̼��ԭ���Թܵ�ú��Ϊȼ�ϵļ�ͥ��Ҫ������Ȼ������ߵĸĽ�������Ϊ
AD
AD
��ѡ����ĸ����
A����������Ľ����� B��������Ȼ���Ľ�����
C����С�����Ľ����� D����С��Ȼ���Ľ�����
��2��ˮ������֮Դ���������úͱ���ˮ��Դ�������岻�ݴǵ����Σ�
���跨��ȥӲˮ�е�
�ơ�þ������
�ơ�þ������
������ʹӲˮ��������ˮ��
�ڹ������������á�����̿+����Ĥ+�����ߡ���Ϲ��ջ��ֱ��ˮ�����л���̿��Ҫ��
����
����
���ã�
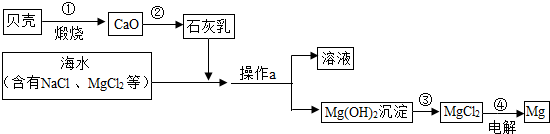
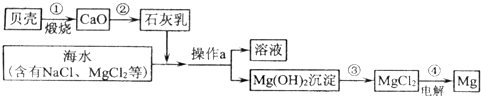
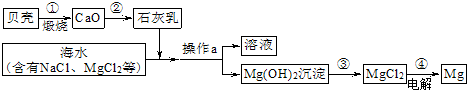
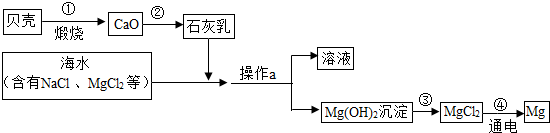
��3����ˮ���д����������õĻ�ѧ��Դ�������������Ȼ�þ�ǽ���þ����Ҫ��Դ֮һ���Ӻ�ˮ����ȡ����þ���ɰ���ͼ���̽��У�

�������й�˵����ȷ����
B
B
��ѡ����ĸ����
A�������ͨ��һ����Ӧ����ʵ�� B���������Ŀ���ǴӺ�ˮ���ᴿ�Ȼ�þ
C��������л�ѧ��ת��Ϊ���� D���ڴ��������漰�Ļ�����Ӧ������4��
���ڴ������п���ѭ�����õ�������
HCl
HCl
��



 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�