
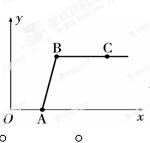
���ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���ijͬѧ������Һʱ��������ʵ�飺ȡ��Һ�����ϲ���Һ�����������ձ��У���ε���Σ����ã�����Һ����������¼����Σ����ã�����Һ�������������й����ı仯��ϵ��ͼ��ʾ�������ж���ȷ���� �� ��
A��ͼ�������꣨������ʾ���ɣã���������
B����Ӧ���е��µ�ʱ����Һ�е������ǣΣ��l
C���ϣ��η�����Ӧ�Ļ�ѧ����ʽ�ǣΣ����ã��������ȣã죽���Σ�ã죫�����ϣ��ã�������
D���õ���Һ�ģ�ȣ�����
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
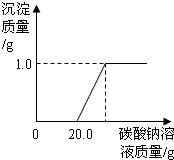 ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ���ϲ���Һ20.0g���ձ��У���ε���������������Ϊ5.3%��̼������Һ������������̼������Һ������/g�������ɳ�����������/g���ı仯��ϵ��ͼ��ʾ������������ȷ��0.1%��
ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ���ϲ���Һ20.0g���ձ��У���ε���������������Ϊ5.3%��̼������Һ������������̼������Һ������/g�������ɳ�����������/g���ı仯��ϵ��ͼ��ʾ������������ȷ��0.1%���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
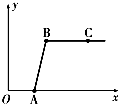 ���ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���ijͬѧ������Һʱ��������ʵ�飺ȡ��Һ�����ϲ���Һ���ձ��У���ε���Na2CO3��Һ����������¼����Na2CO3��Һ������x�����й����ı仯��ϵ��ͼ��ʾ�������ж���ȷ���ǣ�������
���ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���ijͬѧ������Һʱ��������ʵ�飺ȡ��Һ�����ϲ���Һ���ձ��У���ε���Na2CO3��Һ����������¼����Na2CO3��Һ������x�����й����ı仯��ϵ��ͼ��ʾ�������ж���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?��̨����ģ�����ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д����������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС��ͬѧ����������ʵ�飺ȡ��ҺͰ���ϲ���Һ522g������������CaCO3��ĩ������22g���壬���ˣ��õ�a g��Һ������Һ�м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ���������ϣ�Na2CO3+CaCl2=CaCO3��+2NaCl��
��2013?��̨����ģ�����ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д����������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС��ͬѧ����������ʵ�飺ȡ��ҺͰ���ϲ���Һ522g������������CaCO3��ĩ������22g���壬���ˣ��õ�a g��Һ������Һ�м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ���������ϣ�Na2CO3+CaCl2=CaCO3��+2NaCl���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
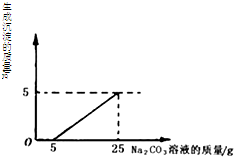 ���ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ��ͬѧ�Ǿ������ø÷�Һ���ⶨһ��Na2CO3��Һ�����ʵ������������������Һ�������μ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ��
���ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ��ͬѧ�Ǿ������ø÷�Һ���ⶨһ��Na2CO3��Һ�����ʵ������������������Һ�������μ�Na2CO3��Һ������Na2CO3��Һ�����������ɳ��������Ĺ�ϵ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����ģ�⣩�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��Һ10kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ����֪��Na2CO3+CaCl2=CaCO3��+2NaCl����
��2013?����ģ�⣩�ڻ�ѧʵ�鼼�ܿ������ꡰ������̼����ȡ�����ʡ�ʵ���ҺͰ���д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ������Ⱦ�������������÷�Һ����ѧ��ȤС����������ʵ�飺ȡ��Һ10kg�������м���������������Ϊ21.2%��̼������Һ��������ҺpH������̼������Һ��������ϵ��ͼ��ʾ����֪��Na2CO3+CaCl2=CaCO3��+2NaCl�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com