
| ��Ҫ�ɷ֣�mg/L���� ̼�������HCO3-����173-205 �����ӣ�Cl-����1.0-8.0 �������SO42-���� 16.08-19.52 �����ӣ�Na+����8-50 PHֵ��7.8±0.5 |


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ��Ҫ�ɷ֣�mg/L���� ̼�������HCO3-����173-205 �����ӣ�Cl-����1.0-8.0 �������SO42-����16.08-19.52 �����ӣ�Na+����8-50 þ���ӣ�2.5-12.5 PHֵ��7.8��0.5��1��þ���ӻ�ѧ���ſɱ�ʾΪ Mg2+ ����SO42-�������֡�2���ĺ�����һ����������Ӵ�������λ�ĸ���� ����2���ÿ�Ȫˮ�� �� �ԣ���ᡱ��������С�������3���ճ��������� ����ˮ ������ˮ��Ӳˮ����ͨ����� ��������ˮ��Ӳ�ȣ�
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� 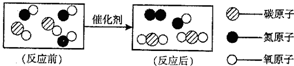 ��1����ͼ��ת������β�����к��������ʾ��ͼ�� ��1����ͼ��ת������β�����к��������ʾ��ͼ���ٷ�Ӧ���ͼʾ�к��� 3 3 �ַ��ӣ���ͼ����ʾ�Ļ�ѧ����ʽ 2CO+2NO
2CO+2NO ��
�۴�ͼ���㻹�ܻ�ȡ����Ϣ�� �ڻ�ѧ�仯�з��ӿ����ٷ֣���ѧ��Ӧǰ��Ԫ�ص�����䣬�������ԭ�ӹ��ɣ��������� �ڻ�ѧ�仯�з��ӿ����ٷ֣���ѧ��Ӧǰ��Ԫ�ص�����䣬�������ԭ�ӹ��ɣ��������� ������һ�֣���2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ�
���ճ������г���______������ˮ��Ӳˮ�� ��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ����______�� �鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ��2012��㶫ʡ÷����ѧ�п���ѧģ���Ծ��������棩 ���ͣ������ ��1����ͼ��ת������β�����к��������ʾ��ͼ�� �ٷ�Ӧ���ͼʾ�к��� �ַ��ӣ� ��ͼ����ʾ�Ļ�ѧ����ʽ �� �۴�ͼ���㻹�ܻ�ȡ����Ϣ�� ������һ�֣� ��2��ij��Ȼ��Ȫˮ����Ҫ�ɷ����±����������Ķ�����գ�
���ճ������г��� ������ˮ��Ӳˮ�� ��ũҵ���ֽ���ֲ��ʱ������ˮ�����Ϊ��ࡢ�ι��Ŀ���� �� 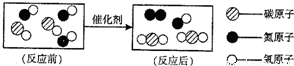 �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |