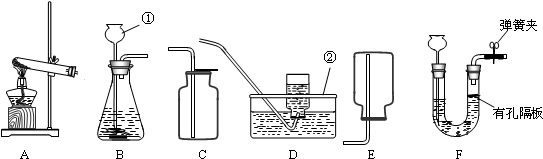
½ā£ŗ£Ø1£©Ķ¼ÖŠ¢ŁŹĒŹŌ¹Ü£¬¢ŚŹĒ³¤¾±Ā©¶·£®
¹Ź“š°øĪŖ£ŗŹŌ¹Ü£»³¤¾±Ā©¶·£»
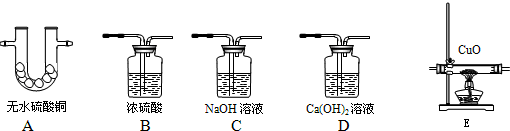
£Ø2£©ŹµŃéŹŅ³£ÓĆŠæĮ£µÄĻ”ĮņĖį·“Ó¦ÖĘČ”ĒāĘų£¬ŹĒ¹ĢŅŗ²»¼ÓČČ·“Ó¦£¬ĖłŅŌæÉŃ”ÓĆB×÷ĪŖ·¢Éś×°ÖĆ£»ĒāĘųµÄĆܶȊ”ÓŚæÕĘųµÄĆÜ¶Č£¬²¢ĒŅ²»Ņ×ČÜÓŚĖ®£¬æÉÓĆCÅÅĖ®·ØŹÕ¼Æ»ņEĻņĻĀÅÅæÕĘų·ØŹÕ¼Æ£®
¹Ź“š°øĪŖ£ŗB£»C»ņE£»
£Ø3£©ŹµŃéŹŅÓĆĀČĖį¼ŲŌŚ¶žŃõ»ÆĆĢµÄ“ß»Æ×÷ÓĆĻĀ¼ÓČČÖĘČ”ŃõĘųµÄ»Æѧ·“Ó¦Ź½ŹĒ£ŗ2KClO
3
2KCl+3O
2”ü£»
¹Ź“š°øĪŖ£ŗ2KClO
3
2KCl+3O
2”ü£»
£Ø4£©Ź×ĻČĢį“棬ČܽāĪļÖŹ£¬Ķعż¹żĀĖ·ÖĄė³ö¶žŃõ»ÆĆĢ¹ĢĢ壬ŌŁĶعżÕō·¢½į¾§”¢øÉŌļµĆµ½ĀČ»Æ¼Ų¾§Ģå£»Č»ŗóŌŁ¼ĘĖć”¢³ĘĮ攢ČܽāÅäÖĆČÜŅŗ£¬ĖłŅŌÕżČ·µÄĖ³ŠņŹĒ£ŗ¢Ł¢Ū¢Ż¢Ś¢Ü¢Ł£»
¹Ź“š°øĪŖ£ŗ¢Ł¢Ū¢Ż¢Ś¢Ü¢Ł£»
£Ø5£©ŌŚÕō·¢ŹµŃéÖŠ£¬ĪŖ·ĄÖ¹ŅŗĢå¾Ö²æŹÜČČ·É½¦£¬ŅŖÓĆ²£Į§°ō½Į°č£®
¹Ź“š°øĪŖ£ŗ½Į°č£»
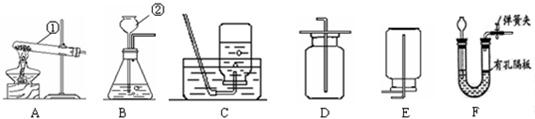
£Ø6£©ČōÓĆ×°ÖĆFÖĘČ”¶žŃõ»ÆĢ¼£¬²¢ÄÜæŲÖĘ·“Ó¦½ųŠŠ»ņĶ£Ö¹£®¹ĢĢåŹÆ»ŅŹÆÓ¦·ÅŌŚÓŠæ×øō°åÉĻ£¬æÉŹ¹·“Ó¦Äܹ»æŲÖĘ£»ĪŖ·ĄÖ¹Éś³ÉµÄĘųĢåŅŻ³ö£¬³¤¾±Ā©¶·ĻĀ¶Ė¹ÜæŚ²åČėŅŗĆęĻĀŠĪ³É·āŅŗ£¬»Æѧ·“Ó¦Ź½ŹĒ£ŗCaCO
3+2HClØTCaCl
2+H
2O+CO
2”ü£®
¹Ź“š°øĪŖ£ŗÓŠæ×øō°åÉĻ£»Ņŗ·ā£»CaCO
3+2HClØTCaCl
2+H
2O+CO
2”ü£®
·ÖĪö£ŗ£Ø1£©ŹģĻ¤³£¼ūŅĒĘ÷£¬ĮĖ½āĆū³Ę£»
£Ø2£©“ÓÖĘČ”ĒāĘųµÄ·“Ó¦ĪļדĢ¬ŗĶ·“Ó¦Ģõ¼žŃ”Ōń·¢Éś×°ÖĆ£¬“ÓĒāĘųµÄĆܶČŗĶČÜĖ®ŠŌæ¼ĀĒŹÕ¼Æ×°ÖĆ£»
£Ø3£©Źģ¼ĒÓĆĀČĖį¼Ų”¢¶žŃõ»ÆĆĢĪŖŌĮĻÖĘČ”O
2µÄ»Æѧ·½³ĢŹ½£»
£Ø4£©øł¾ŻĢį“æĪļÖŹµÄ»ł±¾ŹµŃé²½Öč£ŗ£ŗ¢ŁČܽā¢Ś¹żĀĖ¢ŪÕō·¢¢Ü¼ĘĖć¢Ż³ĘĮ梎ÅäÖĘĄ“½ā“š£»
£Ø5£©Óɲ£Į§°ōµÄ»ł±¾µÄÓĆĶ¾£ØÓĆÓŚ¹żĀĖ”¢½Į°č»ņ×ŖŅĘŅŗĢ壩Ą“»Ų“šĪŹĢā£»
£Ø6£©“ÓæÉæŲÖĘ·“Ó¦µÄĖŁĀŹŗĶ·ĄÖ¹ĘųĢåŅŻ³öæ¼ĀĒ£»Źģ¼ĒŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·“Ó¦Ź½£®
µćĘĄ£ŗÅäÖĘČÜŅŗ³£¼ū²Ł×÷£ŗ¹ĢĢåČÜÖŹ¼ÓĖ®Čܽā£¬ÅäÖĘ²½Öč¼ĘĖć”¢³ĘĮ攢Čܽā£»“ÖŃĪĢį“æµÄŅ»°ć²½ÖčĪŖČܽā”¢¹żĀĖ”¢Õō·¢£®

 2KCl+3O2”ü£»
2KCl+3O2”ü£» 2KCl+3O2”ü£»
2KCl+3O2”ü£»