
| ||


 ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
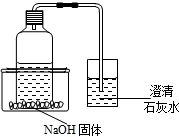 ��2013?�Թ�����̽���Ĵϴ�����һ����ͥСʵ�飺��ʢ�в���ѩ�̣�̼�����ϣ�������ƿ��ƿ���ϲ���һ�������ܣ����ܵ���һ������װ����������ʯ��ˮ�ı����������ƿ����һװ��NaOH����������ڣ�Ȼ���������ڻ���ע��һ������ˮ������ͼ��ʾ��
��2013?�Թ�����̽���Ĵϴ�����һ����ͥСʵ�飺��ʢ�в���ѩ�̣�̼�����ϣ�������ƿ��ƿ���ϲ���һ�������ܣ����ܵ���һ������װ����������ʯ��ˮ�ı����������ƿ����һװ��NaOH����������ڣ�Ȼ���������ڻ���ע��һ������ˮ������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ��Ĵ��Թ�������ѧ�������棩 ���ͣ�̽����
��̽���Ĵϴ�����һ����ͥСʵ�飺��ʢ�в���ѩ�̣�̼�����ϣ�������ƿ��ƿ���ϲ���һ�������ܣ����ܵ���һ������װ����������ʯ��ˮ�ı����������ƿ����һװ��NaOH����������ڣ�Ȼ���������ڻ���ע��һ������ˮ����ͼ��ʾ��
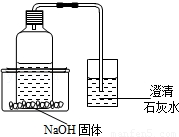
��ش𣺣�1�������е�������������ʯ��ˮ����ǡ����䷴Ӧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O����
��2��˵����NaOH�����ܽ�ʱ�� ����������š����ȣ�CO2���ܽ�����¶ȵ��� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ͥСʵ�飺��ϴ�����鼦���ǣ���Ҫ�ɷ���̼��ƣ�����С�������У�Ȼ�����������ϡ���ᣬ�۲쵽�����DZ��� ���÷�Ӧ�Ļ�ѧ����ʽ�� ��������պ�г���ʯ��ˮ�IJ���Ƭ��ס��һ�������ɹ۲쵽����ʯ��ˮ ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��2����ʢ����ɫʯ����Һ���Թ���ͨ��������CO2����ɫʯ����Һ����Һ��Ϊ ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ�� ������Ӧ�����Һ���ȣ�����Һ��Ϊ ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com