

���� ��1���������ʵ���ɷ������ʵ����
��2�����ݻ�ʯȼ�ϵ����ࡢ��Ȼ���ijɷַ����ش�
��3�����ݻ��ϼ�ԭ�����Ԫ�صĻ��ϼۣ�
��4�����ݽ��ʯ����ȡд����Ӧ�Ļ�ѧ����ʽ��
��5�����ݡ�̼���ࡱ�����ʡ�Ӧ�÷����жϣ�
��6�����ݱ�ȩ���ӵĹ��ɷ�����
��� �⣺��1��̼��ơ�һ����̼���京��̼Ԫ�أ�������ͬ�������ƣ�����Ϊ�����࣮�Ҵ���C2H5OH���Ǻ��к�̼Ԫ�صĻ���������л��
��2����ʯȼ����Ҫ����ú��ʯ�ͺ���Ȼ�������Ƕ�����̼Ԫ�أ�������Ȼ������Ҫ�ɷ��Ǽ��飬��ѧʽ�ǣ�CH4��
��3�������ڻ��������������ϼ۵Ĵ�����Ϊ0����MnCO3�У�̼����Ļ��ϼ�Ϊ-2�ۣ�����Ƴ��̵Ļ��ϼ�Ϊ+2��
��4����440���ѹ�����£��������������̼��Ӧ�����ɽ��ʯ��C����̼���ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��4Na+3CO2$\frac{\underline{\;440���ѹ\;}}{\;}$2Na2CO3+C��
��5�����ڡ�̼���ࡱ�����ж�ṹ�����������ԣ�����ʯ���к�ǿ�������������ɴ�������ʯ��й©
���������ʯ�ͼ������Կɻָ�ԭ״�����ظ�ʹ�ã�
��6���ڱ�ȩ�У����۽Ƕȣ�ÿ����ȩ�����к���10��ԭ�ӣ���Ԫ����ɽǶȣ������ʺ���̼���⡢��3��Ԫ�أ�
�ʴ�Ϊ����1��B����2��ʯ�ͣ�CH4����3��+2����4��4Na+3CO2$\frac{\underline{\;440���ѹ\;}}{\;}$2Na2CO3+C����5��ABC��6��10��3��
���� ������Ҫ�����˺���̼Ԫ�صĵ��ʡ��������֪ʶ���ѶȲ������ڻ�����֪ʶ���������е�֪ʶ������ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ����� | 1 | 2 | 3 | 4 |
| ÿ�μ�����Ʒ������/g | 5 | 5 | 5 | 5 |
| ��Ӧ��ʣ�����ʵ�������/g | 83.9 | 87.8 | m | 97.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ�鷽�� | ʹ�õķ������� �� | �жϵķ��� |
| A | pH��ֽ | ���pH��7�����������Ѿ����� |
| B | ̼���Ʒ�ĩ | ��������ݲ��������������Ѿ����� |
| C | ��������Һ | ����а�ɫ�������������������Ѿ����� |
| D | ��ɫʯ����Һ | �����Һ��ɺ�ɫ�����������Ѿ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
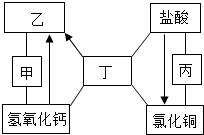 ��ͼ�мס��ҡ��������dz��л�ѧ�г��������ڲ�ͬ�������ʣ�ͼ�С�-����ʾ����������֮���������Һ�з�����ѧ��Ӧ����������ʾ��ij�����ʿ�ͨ��һ����Ӧֱ��ת��Ϊ��һ�����ʣ����ַ�Ӧ������P��Ӧ��������ȥ��������˵������ȷ���ǣ�������
��ͼ�мס��ҡ��������dz��л�ѧ�г��������ڲ�ͬ�������ʣ�ͼ�С�-����ʾ����������֮���������Һ�з�����ѧ��Ӧ����������ʾ��ij�����ʿ�ͨ��һ����Ӧֱ��ת��Ϊ��һ�����ʣ����ַ�Ӧ������P��Ӧ��������ȥ��������˵������ȷ���ǣ�������| A�� | ������ֻ���������� | |
| B�� | �ס��ҡ�����������������������Ϊ���������ʡ��� | |
| C�� | ��ͭԪ�ص����ʶ�����ϡ����ͨ��һ����Ӧֱ�������Ȼ�ͭ | |
| D�� | �ҡ���֮�������˫��ת����ϵ�����ҡ����ֱ��ϡ���ᷴӦ�ܹ�����ͬһ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ȼ�ճ� | B�� |  �ձ� | C�� |  ������ | D�� |  �Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳ�︯�� | B�� | �������� | C�� | ���ͻӷ� | D�� | ���ͨ�緢�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

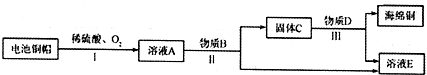
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ڳ�ʪ�Ŀ��������� | B�� | �������ۻ�����ˮ | ||
| C�� | ��ɰֽ��ĥ����Ʒ������� | D�� | ұ��ʱ������ʯ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com