�⣺
��1��ʶ���������ʴ�Ϊ�ƾ��ƣ�����ƿ��
��2������������ķ�Ӧ�ĵ�һ�����Ǽ���װ�õ������ԣ��ʴ�Ϊ�����ԣ�
��3������������ʽֻ�е�һ�������ȣ���������Ϣ-���ܿ�֪���ʴ�Ϊ�٣�����ʽ�ڽ̲��п����ҵ����ʴ�Ϊ2H
2O
2
2H
2O+O
2��
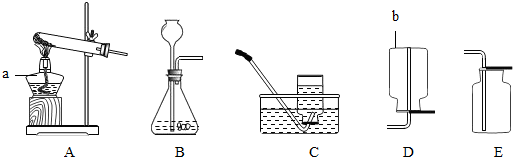
��4������̼��ƺ������ƶ�����̼���ڹ����Һ�岻������ȡ���壬�ʴ�ΪB�����ڶ�����̼���ܶȱȿ�������˲��������ſ��������ʴ�ΪE�����������̼���ķ����ǹ̶���֪ʶ���ʴ�Ϊ��ȼ�ŵ�ľ���쵽����ƿ�ڣ��۲��Ƿ�Ϩ��
CaCO
3����Է�������Ϊ40+12+16��3�T100
�����ɶ�����̼������ΪX
CaCO
3+2HCl�TCaCl
2+H
2O+CO
2��
100 44
150��80% X
��

=

��X�T52.8
�ʴ�Ϊ100��52.8
��������1��ʶ��һЩ����������
��2������ʵ������к���Ҫ��һ����ֻҪ������ķ�Ӧ���ȼ���װ�������ԣ�
��3�����������Ϣ-���ܡ�������֪�𰸣�ֻ�е�һ�����̲��ü��ȣ�����ʽ�ڽ̲��г�����
��4��������̼����ȡ����ʽ������װ�á��ռ�װ���ڽ̲ĺܳ�����Ҳ����Ҫ�����ݣ�������̼����������Ҫ��ס��
����һ����С�ļ����⣬ֻҪд����ѧ����ʽ��Ȼ���̼��Ƶ�����������㼴�ɣ�
���������⿼�������ĺͶ�����̼����ȡװ�ú��ռ�װ�ã��Լ����û�ѧ����ʽ���㣻Ҫ��������ȡװ�ú��ռ�װ�õ�ѡ����

 2H2O+O2��
2H2O+O2�� =
=


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
