
��֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮��������ת����ϵ����ش��������⡣

��1��д��X�ڵ�ȼ�������·�Ӧ����Y�Ļ�ѧ����ʽ ��
��2��д��Yͨ������̼�㷴Ӧ����X�Ļ�ѧ����ʽ ��
��3��д����Z����һϵ�з�Ӧ�����ռ�Ļ�ѧ����ʽ��
�� ��
��4������֪�������ʵ������ṹ�������ʵ����ʡ�����Ҫ�Ļ�ѧ˼�롣����X��Y������ Ԫ�غ�________Ԫ�أ������ǵ��������ʡ���ѧ���ʶ���ͬ����ٳ�X��Y���ʲ�ͬ��һ������ ��
��1�� 2CO��O2 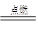 2CO2 ��2�� CO2+C
2CO2 ��2�� CO2+C 2CO
2CO
��3�� CaCO3  CaO��CO2��
��
CaO��H2O=== Ca(OH)2
��
CaO��CO2��
��
CaO��H2O=== Ca(OH)2
��
Ca(OH)2��Na2CO3 === CaCO3����2NaOH
��4�� C����̼����O���������� һ����̼�ж���������̼��
��������X���ж��Ҳ�����ˮ�����壬��X������һ����̼��Y�Dz�֧��ȼ�յ����壬��Y�����Ƕ�����̼��Z�Dz�����ˮ�Ĺ��壬��Z������̼��ơ�
��1��XΪCO��YΪCO2���ʸû�ѧ���̹�ҵΪ2CO��O2 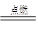 2CO2 ��
2CO2 ��
��2��������̼��̼�ڸ����¿�������һ����̼���ʷ���ʽΪCO2+C 2CO��
2CO��
��3��̼����ڸ����·ֽ����������ƺͶ�����̼�������ƿ�����ˮ��Ӧ�����������ƣ�
��������̼���Ʒ�Ӧ��������̼��ƺ��ռ��������ƣ�����ʽΪCaCO3  CaO��CO2����CaO��H2O=== Ca(OH)2 ��Ca(OH)2��Na2CO3 === CaCO3����2NaOH
CaO��CO2����CaO��H2O=== Ca(OH)2 ��Ca(OH)2��Na2CO3 === CaCO3����2NaOH
��4��һ����̼�Ͷ�����̼��Ԫ�������ͬ��������̼Ԫ�غ���Ԫ����ɵģ����Ƿ��ӹ��ɲ�ͬ��������ʲ�ͬ������һ����̼�ж�����������̼����


 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
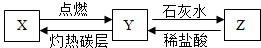 ��2011?��������ģ����֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮��������ת����ϵ��
��2011?��������ģ����֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮��������ת����ϵ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʯ��ˮ |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
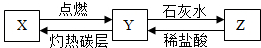
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ȼ |
| ����̼�� |
| ʯ��ˮ |
| ϡ���� |
| ||
| ||
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
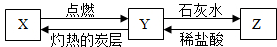 ��2013?����һģ����֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮������ͼ��ʾ��ת����ϵ��
��2013?����һģ����֪X���ж��Ҳ�����ˮ�����壬Y�Dz�֧��ȼ�յ����壬Z�Dz�����ˮ�Ĺ��壬X��Y��Z֮������ͼ��ʾ��ת����ϵ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com