
��ÿ��2�֣���12�֣���ѧ�κ�ѧ��ȤС���ͬѧ������ʵ����ʱ��������һƿ����������Һû������Ƥ����������ʦͬ���չ������̽����
[�������1] ������������Һ�Ƿ�������أ�
[ʵ��̽��1]
| ʵ����� | ʵ������ | ʵ����� |
| ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ������ð���� | ����������Һһ�������ˡ� |
[�������2] ������������Һ��ȫ�����ʻ��Dz��ֱ����أ�
[���������]
����1������������Һ���ֱ��ʡ�
����2������������Һȫ�����ʡ�
[��������] ��1���Ȼ�����Һ�����ԡ�
��2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl
[ʵ��̽��2]
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | �� ���ɡ� | ��ԭ��Һ��һ����̼���ơ� |
| ��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ���� �� |
[ʵ�����] ������������Һ ������֡���ȫ���������ʡ�
[��˼������] ��1������������Һ¶���ڿ��������ױ��ʣ���д����ط�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������[ʵ��̽��2]�У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷���
�����������������
[������Ӧ��]����������Һ���ױ��ʣ������ܷⱣ�档ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬������һ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ÿ��2�֣���12�֣�2011��3��11�գ��ձ�������һ�˵�վ���������й©��
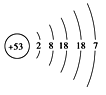
��1����й©�к��е⡪131���ӣ���ԭ�ӽṹʾ��ͼ����ͼ��ʾ����ԭ�ӵĺ˵����Ϊ �����Ӳ���Ϊ �㣻������ Ԫ�أ���������ǽ���������
��2���˵�վ������ȴ�����ĺ���ˮ���ѷ����뺣����繫˾���á�ˮ��������ס��©�㣬��ˮ����������Ҫ�ɷ��ǹ����ƣ�Na2SiO3����
�ٹ������й�Ԫ�صĻ��ϼ��� �ۡ�
�ڶ����������������Ʒ�Ӧ���ɹ����ƺ�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ ���䷴Ӧԭ�������ѧ���� ���������Ʒ�Ӧ���ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�ij��и��ƾ��꼶��һѧ����ĩѧҵ���Ի�ѧ�Ծ� ���ͣ�̽����
��ÿ��2�֣���12�֣���ѧ�κ�ѧ��ȤС���ͬѧ������ʵ����ʱ��������һƿ����������Һû������Ƥ����������ʦͬ���չ������̽����
[�������1] ������������Һ�Ƿ�������أ�
[ʵ��̽��1]
|
ʵ����� |
ʵ������ |
ʵ����� |
|
ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� |
�������� |
����������Һһ�������ˡ� |
[�������2] ������������Һ��ȫ�����ʻ��Dz��ֱ����أ�
[���������]
����1������������Һ���ֱ��ʡ�
����2������������Һȫ�����ʡ�
[��������] ��1���Ȼ�����Һ�����ԡ�
��2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl
[ʵ��̽��2]
|
ʵ�鲽�� |
ʵ������ |
ʵ����� |
|
��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� |
�� ���ɡ� |
��ԭ��Һ��һ����̼���ơ� |
|
��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� |
��Һ���ɫ�� |
˵��ԭ��Һ��һ���� �� |
[ʵ�����] ������������Һ ������֡���ȫ���������ʡ�
[��˼������] ��1������������Һ¶���ڿ��������ױ��ʣ���д����ط�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������[ʵ��̽��2]�У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷���
�����������������
[������Ӧ��]����������Һ���ױ��ʣ������ܷⱣ�档ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬������һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ�ij��и��ƾ��꼶��һѧ����ĩѧҵ���Ի�ѧ�Ծ� ���ͣ������
��ÿ��2�֣���12�֣�2011��3��11�գ��ձ�������һ�˵�վ���������й©��
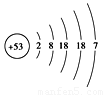
��1����й©�к��е⡪131���ӣ���ԭ�ӽṹʾ��ͼ����ͼ��ʾ����ԭ�ӵĺ˵����Ϊ �����Ӳ���Ϊ �㣻������ Ԫ�أ���������ǽ���������
��2���˵�վ������ȴ�����ĺ���ˮ���ѷ����뺣����繫˾���á�ˮ��������ס��©�㣬��ˮ����������Ҫ�ɷ��ǹ����ƣ�Na2SiO3����
�ٹ������й�Ԫ�صĻ��ϼ��� �ۡ�
�ڶ����������������Ʒ�Ӧ���ɹ����ƺ�ˮ����д���÷�Ӧ�Ļ�ѧ����ʽ ���䷴Ӧԭ�������ѧ���� ���������Ʒ�Ӧ���ơ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com