
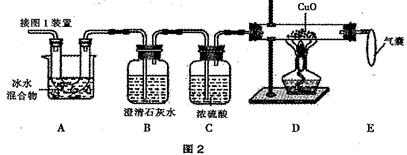
��ѧ�κ�ѧ��ȤС���ͬѧ������ʵ����ʱ��������һƿ����������Һû������Ƥ����������ʦͬ���չ������̽����
[�������1]������������Һ�Ƿ�������أ�
[ʵ��̽��1]
| ʵ����� | ʵ������ | ʵ����� |
| ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ������ð���� | ����������Һһ�������ˣ� |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | ���� �����ɣ� | ˵��ԭ��Һ��һ����̼���ƣ� |
| ��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ������ ���� |
[ʵ��̽��2]����ɫ���������������ƣ���NaOH����[ʵ�����] ���֣�[��˼������]��1��CO2+2NaOH=Na2CO3+H2O����2�������У�[������Ӧ��]�ӷ��Ե�ҩƷ��Ũ���ᡢ��ˮ�ȣ�������ˮ�Ե�ҩƷ��Ũ���ᡢ��ʯ�ҡ������ʯ��ˮ���������𰸾��ɣ�
���������������һƿû������Ƥ��������������Һ����̽������[ʵ��̽��1]֪����������Һ�ѱ��ʣ�����������Һ�к���̼������ӣ�[��������]��1���Ȼ�����Һ�����ԣ���2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl������[ʵ��̽��2]����1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ�����������а�ɫ�������ɣ�˵��ԭ��Һ��һ����̼���ƣ���2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ����Һ���ɫ��˵��ԭ��Һ��һ�����������ƣ��Ӷ��ó�[ʵ�����]������������Һ���ֱ��ʣ���������������Һ�����Ȼ�����Һ�Dz��еģ�������ȷ����2�������ʵ����ۣ��ò����������Ʋ��ֱ��ʵĽ��ۣ�����������Һ¶���ڿ��������ױ��ʣ�д����ط�Ӧ�Ļ�ѧ����ʽ��CO2+2NaOH=Na2CO3+H2O�����[������Ӧ��]����������Һ���ױ��ʣ������ܷⱣ�森ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬�磺�ӷ��Ե�ҩƷ��Ũ���ᡢ��ˮ�ȣ�������ˮ�Ե�ҩƷ��Ũ���ᡢ��ʯ�ҵȣ��������ʯ��ˮ���������𰸾��ɣ�
���㣺ҩƷ�Ƿ���ʵ�̽������������ʵ��֤����Ļ�ѧ���ʣ�̼���ơ�̼��������̼��ƣ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
��ѧ�������ҵ�����������ÿ�����CO2�Ĺ��룺�ѿ������뱥��̼������Һ�У���Һ������CO2����̼�����ƣ�����̼�����ƹ����ַֽ�ų�CO2���ںϳ�����CO2��������Ӧ���ɼ״���CH3OH����ˮ����Ҫ��������������ͼ��ʾ��
[���Ͽ�Ƭ] ̼�����Ʒֽ��¶���270�棬̼������856���ۻ�������δ�ﵽ�ֽ��¶ȡ�
��ش��������⣺
��1�����ճ��з����˻��Ϸ�Ӧ����ѧ����ʽΪ ��
��2���������ÿ����е�CO2�����ʹ�����CO2Ũ�ȣ������ڼ��� ��
��3���ϳ����з�����Ӧ�Ļ�ѧ����ʽΪ ��
��4������������ѭ�����õ������� ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ij�о�С����������ᾧ����Ʒ�ֽ������ⶨ��Ʒ�в��ᾧ��������������������ʲ����뷴Ӧ������֪��Ũ�������Ϊ����������ᾧ�壨H2C2O4��2H2O �������ʼ��±���
| �۵� | �е� | ���ȶ��� | ���� |
| 101�桫102�� | 150�桫160������ | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca(OH)2��Ӧ������ɫ����(CaC2O4) |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ij��ȤС���ͬѧ����ʦ��ָ���£���һƿû������Ƥ��������������Һ����̽��������һ����룮
��������⡿������������Һ�Ƿ�ȫ�����ʣ�
����������衿����٣�����������Һ����ȫ�����ʣ����̼��ƣ�
����ڣ�����������Һ���ܲ��ֱ��ʣ���ɷ����� ����
��ʵ��̽����
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ�����Թ��У��μ�ϡ���� | ���� ������ | ԭ��Һ��һ����̼��� |
| ��2����ȡ�����Թ��У��μӷ�̪��Һ | ��Һ�ɺ�ɫ | ԭ��Һһ������ �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ijͬѧ��̽������ ��˫�Ʒ��������ࡢ����塱��������۵���Ҫ�ɷ֡�
���������ϡ�
��1����������۾�����Ħ���������Լ������ϵȳɷֹ��ɡ�
��2�����õ�Ħ�����м�ϸ������̼���(CaCO3) ��ˮ�Ϲ���(SiO2��nH2O)��
��3������������г���̼������⣬�������ʾ�����ϡ���ᷴӦ�������塣
��ʵ��һ��̽��������������������Ƿ���̼��ƣ�
| ���� | ʵ����� | ʵ������ |
| �� |  ��ͼ��ʾ��ȡ��ֻ�Թֱܷ��������������Ʒ���ٷֱ��������R ��Һ��R ��Һ�� �� | A�Թ��������Ա仯�� B��C�Թ�������ɫ�������ɡ� |
| �� | ��B��C�Թ������ɵ���ɫ����ͨ�����ʯ��ˮ�� | ����� �� |
| ���� | ʵ����� | ʵ������ |
| �� | ��װ������ͼװ�ý���ʵ�顣�ֱ�ȡ ��������������ƿ�С����ڷ�Һ©���зֱ�����������R ��Һ��  | |
| �� | ��Һ©��ע��һ����R ��Һ��Ȼ��رջ����� | �����ݲ����� |
| �� | ���ڷ�Ӧ�������ٴ�Һ©����ע��һ����R ��Һ��Ȼ��رջ����� | ���������� |
| �� | ������Ӧ��װ�ü�ҩƷ�������������ԱȽ� | ���롰˫�Ʒ����������װ�ü�ҩƷ�����������ڼ������۵�װ�ü�ҩƷ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
�������ijѧϰС���̽��������ش�������⣮
ij��ѧ��ȤС���ͬѧ���������Ƶ�Ƭ״������ڱ������У��뿪ʵ���ң��������й۲죮
�������⣺�����Ƭ״�����ɰ�ɫ��ĩ״������
������⣺����ɫ��ĩ����������ʲô����
�������裺�� ��
���ʵ�飺�������������ʵ�飬�����������д�ڱ����У�
| ʵ�鷽�������� | ���ܹ۲쵽������ | ʵ����� |
| �� �� | �� �� | �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ij��ѧ��ȤС���ͬѧ��һö�ྻ����������ʳ��ˮ�У���ͼ��װ�����������ã���һ��ʱ�����װ�����Ҳർ���е�Һ�����ߣ��������⣬�Թܵײ��к�ɫ��������
[�������]������Һ��Ϊ�����ߣ���ɫ������������ʲô��
[���������]
�ú�ɫ������ܺ��У���Fe����Fe2O3����FeCl3����C��������
[�������]
��Ӱ���Ҳർ���е�Һ�����ߵ�����������
�ڲ����ܺ����Ȼ������������� ��
��һ������̼���������� ��
[ʵ����֤]
����֤��ɫ������һ������̼��ѡ�õ��Լ����� ��
�ڼ����ɫ�������Ƿ�������ʵ�鷽������ ��
���ۣ�������ʳ��ˮ�б��ڴ�ˮ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
̼����ڸ���������һ��ʱ��õ���ɫ���壨CaCO3 CaO+CO2������Ϊ��ȷ����ɫ����ijɷ֣�������ܵ���ɽ���̽������������±���
CaO+CO2������Ϊ��ȷ����ɫ����ijɷ֣�������ܵ���ɽ���̽������������±���
| ���裨���룩 | ��֤���������� | ���ܿ��������� | �� �� |
| | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�̽����
ͬѧ����ѧϰ��ľ̿��ԭ����ͭ��ʵ������뵽����̼��һЩ��ѧ���ʣ��Է�Ӧ�������ɷֲ��������ʣ�ijѧϰС��Ը÷�Ӧ�����е�����ɷֽ�����̽����
���� �롳
���������ȫ����CO2 ���������ȫ����CO ���������
���������ϡ� CO������ʹʪ��Ļ�ɫ�Ȼ�����ֽ����ɫ��
�����ʵ�顳���ݸ��ԵIJ��룬���ǹ�ͬ���������ͼ��ʵ��װ�ý���̽����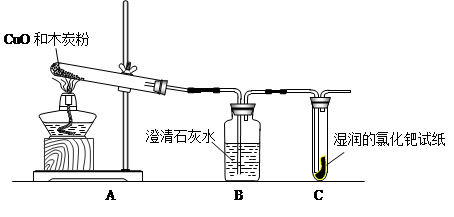
�������������
| ���� | ���� |
| ���װ��B �� װ��C������ | �����ٳ��� |
| ���װ��B������װ��C | �����ڳ��� |
| ���װ��B ��װ��C____________ | �����۳��� |
 2Cu+CO2���е�һ�ֲ���ͷ�Ӧ��ľ̿�ַ����˻�ѧ��Ӧ��������CO��
2Cu+CO2���е�һ�ֲ���ͷ�Ӧ��ľ̿�ַ����˻�ѧ��Ӧ��������CO���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com