
���� ��1�����ݱ�ϩȩ�Ļ�ѧʽ��Ԫ�ص����ԭ�����������Լ������ϩȩ����Է���������
��2�����ݱ�ϩȩ�Ļ�ѧʽ��Ԫ�ص�����������ʽ���������ϩȩ����Ԫ�ص�����������
��3�����ݱ�ϩȩ�Ļ�ѧʽ��Ԫ�ص�����������ʽ�ȼ������ϩȩ��̼Ԫ�ص������������ٸ���̼Ԫ�ص�����=224g����ϩȩ��̼Ԫ�ص������������㼴�ɣ�
��� �⣺
��1�����ݱ�ϩȩ�Ļ�ѧʽ��C2H3CHO����֪��������Է�������Ϊ��12��3+1��4+16=56��
��2����ϩȩ��̼���⡢������Ԫ�ص�������Ϊ����12��3������1��4����16=36��4��16=9��1��4���ʴ�Ϊ��9��1��4��
��3����ϩȩ��̼Ԫ�ص���������Ϊ��$\frac{12��3}{56}��100%$����112g��ϩȩ��̼Ԫ�ص�����Ϊ��112g��$\frac{12��3}{56}��100%$=72g��
�ʴ�Ϊ��
��1��56����2��9��1��4����3��72g��
���� ������Ҫ����ѧ�����û�ѧʽ�����ԭ�������Լ�Ԫ�ص�����������ʽ���м����������


 ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �� Ӧ �� | �� ѧ �� �� ʽ | |
| 1 | �������������ᷴӦ | Ca��OH��2+2HCl=CaCl2+2H2O |
| 2 | ����������ϡ���ᷴӦ | 2NaOH+H2SO4=Na2SO4+2H2O |
| 3 | ��θ��ƽ�������������к����θ�� | Al��OH��3+3HCl=AlCl3+3H2O |
| 4 | ����ʯ���кͺ����������ˮ | Ca��OH��2+H2SO4=CaSO4+2H2O |
| 5 | ̼��������ϡ���ᷴӦ | NaHCO3+HCl=NaCl+H2O+CO2�� |
| 6 | ��ϡ��������� | 6HCl+Fe2O3=2FeCl3+3H2O |
| 7 | ��ϡ��������� | 3H2SO4+Fe2O3=Fe2��SO4��3+3H2O |
| 8 | ��̼������ȡ�������� | Ca��OH��2+Na2CO3=CaCO3��+2NaOH |
| 9 | �Ȼ�����ϡ���ᷴӦ | BaCl2+H2SO4=BaSO4��+2HCl |
| 10 | ������������������Ӧ | 2NaOH+SO3=Na2SO4+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ԭ�ӵ����� | B�� | ������ǧ������λ | ||
| C�� | ���Ǹ���ֵ | D�� | ��ֵ��Լ������������������֮�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
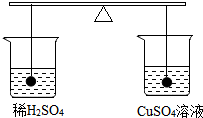 ��ͼ��ʾ�ڴ�С��ͬ����ֻ�ձ��и�װ�е�������ϡ���������ͭ��Һ��Ȼ������������ͭ�ֱ��û������ͭ��Һ��ϡ������Ƭ�̣�����˵����ȷ���ǣ�������
��ͼ��ʾ�ڴ�С��ͬ����ֻ�ձ��и�װ�е�������ϡ���������ͭ��Һ��Ȼ������������ͭ�ֱ��û������ͭ��Һ��ϡ������Ƭ�̣�����˵����ȷ���ǣ�������| A�� | ����ձ�����Һ���������� | B�� | �ұ��ձ�����Һ���������� | ||
| C�� | �����ձ�����Һ������������ | D�� | Ƭ�̺�������Ȼƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2-2����ԭ�� | B�� | 2O-2����Ԫ�� | C�� | P2O5-���������� | D�� | $\stackrel{+2}{Ca}$-������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ɱ������������ | B�� | �ɱ����Ӽ�ļ����� | ||
| C�� | �ɱ����������С | D�� | �ɱ������˶����ʲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� |  ��ɫʯ����Һ�����족 | B�� |  ϡ���ᡰ���족 | ||
| C�� |  ��ɫ��̪��Һ�����족 | D�� |  ˮ�����족 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�ⶼ����ȼ�ϣ���ͬ���ʵķ��ӣ��仯ѧ������ͬ�� | |
| B�� | �þ�Ȯ�Ѿȵ����б�����Ա�������ڲ����˶��� | |
| C�� | ��ˮ���¶ȼƲ������£��¶����ߣ�ԭ�Ӽ������ | |
| D�� | ˮ�տ����װѺ��dz����¶����ߣ����ӱ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com