
ij̼������Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ����Ʒ��̼���Ƶ���������������������ʵ�飺
�� ʵ��һ��
��ش��������⣺
������A���õ����������������� ��
����ͬѧȡ������̼������Ʒ����Һ��������ɫ��̪��Һ����Һ����ɫ����� ��
������ʵ������м��뱥��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
������Ϊ̽��������Ӧ����Һ�е����ʳɷ֣���ͬѧ����Һ�еμӹ���ϡ���ᣬ����
�����ݲ�������μ�����ǰ��Һ�е����ʳ��Ȼ������ ��
�� ʵ�����
��ͬѧȡ12g��̼������Ʒ�����ձ��У�����100gϡ���ᣨ����������ȫ��Ӧ�� ��
����Һ����Ϊ107.6 g���Լ��㣺
�������ɶ�����̼���ʵ���Ϊ mol��
����̼������Ʒ��Na2CO3��������������д��������̣�������0.1%��
���������õĽ����ʵ�ʵ����������ߣ����ܵ�ԭ���� ������һ�ּ��ɣ�
��ʵ��һ
�������� ����ɫ ����Na2CO3 +Ca(OH)2 = 2NaOH +CaCO3��
������̼���ơ��������ƣ�д��ѧʽҲ�ɣ�������©һ�����÷֣�
��ʵ���������0.1mol
����88.3%
����ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ����ˮ������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��1�֣�
�������������̼������Ʒ�к��������Ȼ������ʣ���ˮ�ܽ��õ�̼������Һ���������Ȼ��ƣ���Һ�ʼ��ԣ��ӷ�̪Ӧ���ɫ��̼�����ܺ��������Ʒ�Ӧ����ѧ����ʽΪNa2CO3 +Ca(OH)2 = 2NaOH +CaCO3�����г������ɣ�����A����Ϊ���ˣ�����������Ϊ�������μӹ���ϡ���ᣬ���������ݲ�������һ����̼���ƣ�������������Ҳһ���У���Ϊ������Ӧ�������ɳ�����Ҳ�������������ƣ�������Һ�л����������ƺ�̼���ƣ����������٣����ٵ�������Ϊ���ɶ�����̼��������Ϊ112-107.6=4.4g�����ʵ���Ϊ0.1mol��
Ȼ����ݻ�ѧ����ʽ���㣺����Ʒ�к�̼����Xmol
Na2CO3��2HCl=2NaCl��H2O��CO2��
1mol 1mol
x 0.1g
1��1= x��0.1 x = 0.1mol
��Ʒ��̼���Ƶ���������Ϊ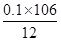 ��100% = 88.3%
��100% = 88.3%
�����õĽ����ʵ�ʵ����������ߣ��������̼����Ӧ��ƫ���ɴ˿��Ƴ�ԭ�����Ϊϡ����ӷ�����HCl������CO2�����ų���ˮ������CO2�����ų������²ⶨ�Ķ�����̼��ƫ�����������̼���Ƶ���������ƫ��
���㣺ʵ��̽�������ݻ�ѧ����ʽ���㣬��Һ�����ʵ��жϣ����ķ����ȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���������б����ش����⣺
��1����֪A��B��C��D����������Ԫ�ء�����A�м���Һ��B���ܷų������ȡ�����C�м���Һ��D�������ݲ�������ַ�Ӧ����ˣ�ֻ�õ�Һ��B����C��
��A��B��Ӧ������������ڸ����������������ڽ������ϵȣ���A�Ļ�ѧʽΪ________��
��C��D������Ӧ�Ļ�ѧ����ʽΪ________��
��2������E��F��Һ��G��H���������У�����һ�ֹ���������һ��Һ���Ͼ������ݲ�����Eͨ��������Ӧ������F��
����EΪ���ʣ�E��F�Ļ�ѧʽ����Ϊ ��дһ�鼴�ɣ���
����E��F��������Ԫ�أ�E����F�Ļ�ѧ����ʽΪ ������E�Ͷ��������ڸ��������¿����ɹ����ƣ�Na2SiO3����һ���ܲ�������ЧӦ�ij������壬�÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪Ba2+һ�����������ս���ѪҺ��ʹѪҺ�еĵ��������ᣬ����ж���Σ��������Ȼ����BaSO4ȴ������X����Ӱ���θ�������ġ����͡���ԭ����BaSO4���ܽ��Լ�С������Ba2+�������������Զ��������Σ����BaCO3Ҳ���������ʣ��������Բ������BaSO4��Ϊ�����͡������������ж���ԭ���û�ѧ����ʽ��ʾΪ �������BaCO3���ɷ���MgSO4���нⶾԭ���û�ѧ����ʽ��ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������NH3����һ����ɫ�д̼�����ζ�����壬��������ˮ������ˮ��Һ��Ϊ��ˮ���Լ��ԡ������ڻ�ѧ��ҵ����;�ܹ㷺�������ƻ��ʡ��ƴ���ȡ������������������ڻ���������
��1���������Ƽ����������Ҫ��Ӧԭ���ɱ�ʾΪ��
��NH3��CO2 ��H2O��NaCl=NaHCO3����NH4Cl��
��2NaHCO3 Na2CO3�� H2O �� CO2�������У�NaHCO3���� �����ᡱ��������Ρ�������Ӧ������ ��Ӧ��Na2CO3�������� ��
Na2CO3�� H2O �� CO2�������У�NaHCO3���� �����ᡱ��������Ρ�������Ӧ������ ��Ӧ��Na2CO3�������� ��
��2����ϸ�������£��ð��������״��Ĺ�ҵ��ˮ��ʹ���Ϊ����N2��CO2���Ӷ�����Ի�������Ⱦ���йصķ�ӦΪ��6NH3 + 5CH3OH + 12B = 3N2��+ 5CO2��+ 19H2O������B���ʵĻ�ѧʽ�� ��
��3���������Ա��������գ����ɿ����������ʵ�����泥��÷�Ӧ�Ļ�ѧ����ʽΪ��2NH3+H2SO4 = (NH4)2SO4�������������հ�������Ӧ�Ļ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ά����C(���Vc����������Ѫ��)��������ˮ���ױ�����������ȱ��Vc�����������ּ�����ˮ�����߲��к��зḻ��Vc��ij�о���ѧϰС�����̽�����£�
̽��һ���ⶨ������Vc�ĺ�����
���������ϡ�Vc�ܺ�����ط�Ӧ��ʹ��ɫ�ĸ��������Һ��ɫ��
����Ʒ������ֱ���ʢ��1 mL��Ũ�ȸ������ϡ��Һ����֧�Թ�����εμӹ�ζ���ϡ�ƻ��֭����֭��0.04%��Vc��Һ���ߵα���ֱ�����������Һ��ɫ��
��ʵ�����ݡ�
| | ��ζ���� | ƻ��֭ | ��֭ | 0.04%��Vc��Һ |
| �μӵĵ��� | 40 | 10 | 20 | 5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ��֤�����ڱ�¶�ڿ����е�����������Һ�Ѿ����ֱ��ʣ���ֱ�ѡ�����ֲ�ͬ���������Լ����֤������Ҫ����д��
(1)ѡ�õ��Լ�Ϊ ��ʵ������Ϊ ��
(2)ѡ�õ��Լ�Ϊ ��ʵ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ڻ������조�����Ļ�֮������ʳ�����������Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
(1)ijѧϰС����д����ᴿʵ�飬��Ҫ�������²������裺�������ܽ��_____���������ٴγ�����������ʡ�������������ʹ�õ���������������_________��
ʵ�������С�鷢������ʳ�β������Ե�������С�飬��ԭ�������_____��
A.����δ����ܽ����
B.�㵹ʱ���в����Ȼ�����Һ����
C.���������õľ��νϳ�ʪ
(2)��ⱥ��ʳ��ˮ���Եõ����ֻ�����Ʒ����Ҫ�������£�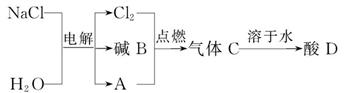
������A���ܶ���С�����壬�����������Ϊ________��д��һ������Dת��Ϊ����A�Ļ�ѧ����ʽ_____________________________________________��
�÷�Ӧ��___________(����ȡ������ȡ�)��Ӧ��
�ڵ������ɵļ�B�Ļ�ѧʽΪ_______��������������_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
С�����˸������ͣ�����һ�����ʡ���Ƥ������ӡ����ͼ��ʾ�ı�ǩ�����ᵽһ�ɳ�ζ��
| ̼�����NH4HCO3 ���أ�50 kg ����������17.38% ������ѧ��ҵƷ��˾ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����к��п��������ʣ��Ȼ�þ���Ȼ��Ƶȣ��Ͳ��������ʡ������ᴿ�IJ����������¡�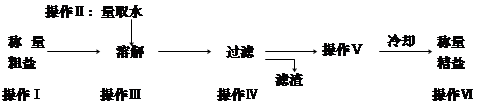
��1�����������г�ȥ�������� ��
��2���������Ŀ���ǣ�a ��b.������ʣ�
��3����ȡˮ��Ҫ�õ��������� ��
��4���������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com