
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����BaCl2 ��Һ������/g | 15 | 20 | 25 | 30 |
| ��Ӧ�õ��ij���������/g | 1.398 | 1.864 | 2.330 | 2.330 |

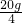 ���ء� 10g��10%
���ء� 10g��10% =
=



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����BaCl2 ��Һ������/g |
15 | 20 | 25 | 30 |
| ��Ӧ�õ��ij���������/g | 1.398 | 1.864 | 2.330 | 2.330 |
| 20g |
| 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007��㶫ʡ��ɽ�и��н�ѧУ�������Ի�ѧ���⼰��(WORD��) ���ͣ�059
С�ú�С������ⶨ���ᡢ������ɵĻ���������ʵ�������������Ʋ���������������̽��ʵ�飺
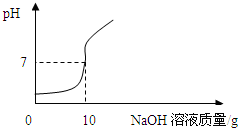
ʵ��1��ȡ20 g�û���Ტ�ֳ�4�ȷ���Ȼ���ֱ�����һ����δ֪����������BaCl2��Һ��ʵ���¼���£�

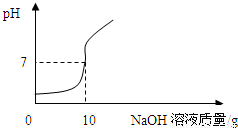
ʵ��2����ʵ��1�еĵ�4�ݷ�ӦҺ���ˣ�������Һ����μ���10����NaOH��Һ��ͨ��pH�ⶨ�Ǵ�ӡ������NaOH��Һ���������ձ�����ҺpH�Ĺ�ϵ����ͼ��

���ݴ�����
С������ʵ��1����������������������H2SO4������������
С������ʵ��2��������HCl�������������������£�
�⣺��������HCl����������Ϊ�أ�
HCl��NaOH��NaCl��H2O

��ã��أ�0.1825��18.25��
���������̽�������������⣮(�����õ�����Է���������H2SO4��98��BaSO4��233��BaCl2��208)
(1)ʵ��1�з�����Ӧ�Ļ�ѧ����ʽΪ________��
(2)С�ò��ԭ�������H2SO4����������Ϊ________(������λ��Ч����)��
(3)С�������������HCl������������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��������___________________(�硰��Ӱ�족�����ղ�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ�п����� ���ͣ�ʵ����



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007��㶫ʡ��ɽ���п���ѧ�Ծ��������棩 ���ͣ������
| ��1�� | ��2�� | ��3�� | ��4�� | |
| ����BaCl2 ��Һ������/g | 15 | 20 | 25 | 30 |
| ��Ӧ�õ��ij���������/g | 1.398 | 1.864 | 2.330 | 2.330 |
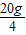 ×�� 10g×10%
×�� 10g×10%�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com