
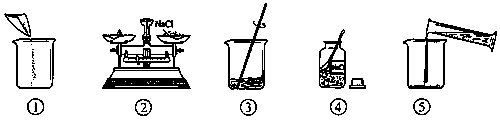
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α��п��桡2009��2010ѧ�ꡡ��19�ڡ���175�� �˽̿α��п��� ���ͣ�058
��ͼ��С������120 g������������Ϊ12����NaCl��Һ���й�ʵ�����ʾ��ͼ��

(1)���ƹ�����ʹ�õ����ֲ����������ֱ��ǹ��ƿ��________��________����Ͳ��
(2)����ָ��ͼ�еĴ���������������ò���������ɵĺ����
________________��
(3)ͼ�ں�ͼ�۱�ʾ�IJ�������ֱ���________��________��
(4)����ʱ������Ȼ���________g������Ȼ����к����������ܵ����ʣ����ʵ�����������________(�ƫ��ƫС��)����ȡˮ���ѡ��________����Ͳ(����Ţ�10 mL����50 mL����100 mL)��(ˮ���ܶ�Ϊ1 g/cm3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ�����г��б�ҵ��ѧ���������Ͼ���ѧ���� ���ͣ�022
��ͼ��С������100 g��������Ϊ18.5����������Һ��ʵ�����ʾ��ͼ��

(1)����������Һ��С��Ҫ��ȡ����________g��
(2)��ͼ�١��ڡ�������ͬ������������________��
(3)����ͼ��ʾ�������ʾ��ȷ���Ƹ���Һ�IJ���˳��Ϊ________��________��________��________��________��
(4)����ͼ�������������Ϊ15 g������Ķ���Ϊ3.5 g����С���Ƶõ���������ʵ��Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007��ɽ��ʡ̫ԭ�г���ѧҵ���������ۺ��Ծ�(��ѧ����)(����) ���ͣ�058
��ͼ��С������100 g������������Ϊ12����NaCl��Һ���й�ʵ�����ʾ��ͼ��

(1)���ƹ�����ʹ�õ����ֲ����������ֱ��ǹ��ƿ��________��________����Ͳ��
(2)����ָ��ͼ��һ������������������ò���������ɵĺ����________��
(3)ͼ�ڡ�ͼ�۱�ʾ�IJ�������ֱ���________��
(4)����ʱ������Ȼ���________g������Ȼ����к����������ܵ����ʣ����ʵ�����������________(�ƫ��ƫС��)����ȡˮ���ѡ��________����Ͳ(����Ţ�10 mL����50 mL����100 mL)��(ˮ���ܶ�Ϊ1 g/cm3)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���½�ά������������½������������2011���п���ѧ���� ���ͣ�058
������С��ͬѧ����100 g��10����NaCl��Һ��ʵ�����ʾ��ͼ��

(1)��ʵ����ȷ�IJ���˳����________(�����)��
(2)����۲�ָ��ͼ�������IJ�������________(�����)��
(3)���Ƹ���Һ��ҪNaCl����________g����Ҫˮ________mL(��ˮ��1 g/mL)��
(4)С��ͬѧ��������ϵ�ʳ��ת�Ƶ��ձ���ʱ������������ʳ�������������ϣ�������ʹ�����Ƶ���Һ��������������________10��(���������������)��
(5)���������ܽ����������ͼ��ʾ����20��ʱ����40 g��NaCl������뵽100 gˮ�У�����ʹ�����ܽ⣬����Ϊ����NaCl��Һ��������________g����������������________��(��ȷ��0.1��)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������С��ͬѧ����100 g 10% NaCl��Һ��ʵ�����ʾ��ͼ��

��1����ʵ����ȷ�IJ���˳����________������ţ���
��2������۲�ָ��ͼ�д���IJ�������________������ţ���
��3�����Ƹ���Һ��ҪNaCl����________g����Ҫˮ________mL����ˮ��1 g/mL����
��4��С��ͬѧ��������ϵ�ʳ��ת�Ƶ��ձ���ʱ������������ʳ�������������ϣ�������ʹ�����Ƶ���Һ��������������________10%���������������������
��5�����������ܽ����������ͼ��ʾ����20 ��ʱ����40 g NaCl������뵽100 gˮ�У�����ʹ�����ܽ⣬����Ϊ����NaCl��Һ��������________g����������������________%����ȷ��0.1%����

����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com