

| m(HCl) |
| m(HClČÜŅŗ) |
| m(HCl) |
| 198.25-m(zn) |
| 65 |
| x |
| 2 |
| 0.5g |
| 50.00g-16.25g |
| 50.00g |
| m(HCl) |
| m(HClČÜŅŗ) |
| m(HCl) |
| 198.25-m(zn) |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
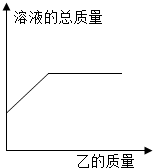 £Ø2013?ŃĪ³ĒÄ£Äā£©ŌŚŅ»¶ØÖŹĮæµÄ¼×ČÜŅŗÖŠÖš½„¼ÓČėŅŅÖĮ¹żĮ棬ŹµŃé¹ż³ĢÖŠČÜŅŗµÄ×ÜÖŹĮæÓė¼ÓČėŅŅµÄÖŹĮæ¹ŲĻµ£¬ÄÜÓĆČēĶ¼ĒśĻß±ķŹ¾µÄŹĒ £Ø””””£© £Ø2013?ŃĪ³ĒÄ£Äā£©ŌŚŅ»¶ØÖŹĮæµÄ¼×ČÜŅŗÖŠÖš½„¼ÓČėŅŅÖĮ¹żĮ棬ŹµŃé¹ż³ĢÖŠČÜŅŗµÄ×ÜÖŹĮæÓė¼ÓČėŅŅµÄÖŹĮæ¹ŲĻµ£¬ÄÜÓĆČēĶ¼ĒśĻß±ķŹ¾µÄŹĒ £Ø””””£©
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2013?ŃĪ³ĒÄ£Äā£©ŹµŃéŹŅ³£ÓƱ„ŗĶŃĒĻõĖįÄĘÓėĀČ»Æļ§ČÜŅŗ·“Ó¦ÖĘČ”“æ¾»µÄµŖĘų£®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNaNO2+NH4Cl=NaCl+N2+2H2O£Ø“Ė·“Ó¦ŹĒ·ÅČČ·“Ó¦£©£¬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£®ŹŌ»Ų“š£ŗ
£Ø2013?ŃĪ³ĒÄ£Äā£©ŹµŃéŹŅ³£ÓƱ„ŗĶŃĒĻõĖįÄĘÓėĀČ»Æļ§ČÜŅŗ·“Ó¦ÖĘČ”“æ¾»µÄµŖĘų£®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗNaNO2+NH4Cl=NaCl+N2+2H2O£Ø“Ė·“Ó¦ŹĒ·ÅČČ·“Ó¦£©£¬ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾£®ŹŌ»Ų“š£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com