
��7�֣�Ϊ�ⶨij��aCl����a2CO3�����������ɣ�С��ͬѧȡ����g�û��������ձ��У�����μ���ϡ���ᣨÿ�μ���ϡ���������Ϊ����g��������Ӧ��ȫ�õ������������ϵ��
| ����ϡ����Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� | ����� |
| �ձ�����Ӧ���������������g | 122.2 | 146.1 | 170.0 | 193.9 | 218.9 |
������������ݺ���㣺
��ԭ���������У�a2CO3��������
�Ƶ�����ϡ��������������ǡ����ȫ��Ӧʱ��������Һ��������������������������ȷ����.����
(1)�ã���������Ϊ��.��g����1�֣�
�������УΣ����ã�������Ϊ�������ɣΣ�ã������Ϊ��
�Σ����ã��������ȣã�=���Σ�ã죫�ã������������ϣ�1�֣�������/��������/��.��g ��1�֣�
�������������������������������������� ��������.��g��1�֣�
���������������������������� ������.��g ������������/��������/��.��g ��1�֣�
����ȫ��Ӧ��������Һ��������Ϊ�� ��������.��g��1�֣�
(����.��g������g������.��g)/(����g������g��������.��g)����������������.������1�֣�
���ԡ�
����:��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?������һģ����ش������йء��߽���̼�������⣺
��2012?������һģ����ش������йء��߽���̼�������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
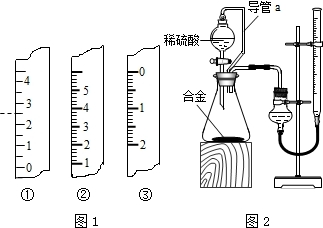
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�콭��ʡ�п�ģ�⿼�Ի�ѧ�Ծ� ���ͣ�������
Ϊ�ⶨij��aCl����a2CO3�����������ɣ�С��ͬѧȡ����g�û��������ձ��У�����μ���ϡ���ᣨÿ�μ���ϡ���������Ϊ����g��������Ӧ��ȫ�õ������������ϵ��
������������ݺ���㣺
��ԭ���������У�a2CO3��������
�Ƶ�����ϡ��������������ǡ����ȫ��Ӧʱ��������Һ��������������������������ȷ����.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�꽭��ʡ�п�ģ�⿼�Ի�ѧ�Ծ� ���ͣ�������
��7�֣�Ϊ�ⶨij��aCl����a2CO3�����������ɣ�С��ͬѧȡ����g�û��������ձ��У�����μ���ϡ���ᣨÿ�μ���ϡ���������Ϊ����g��������Ӧ��ȫ�õ������������ϵ��
|
����ϡ����Ĵ��� |
��һ�� |
�ڶ��� |
������ |
���Ĵ� |
����� |
|
�ձ�����Ӧ���������������g |
122.2 |
146.1 |
170.0 |
193.9 |
218.9 |
������������ݺ���㣺
��ԭ���������У�a2CO3��������
�Ƶ�����ϡ��������������ǡ����ȫ��Ӧʱ��������Һ��������������������������ȷ����.����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com