���㣺��ѧ���ż�����Χ���ֵ�����,������Һ�Ͳ�������Һ,�����ܽ������������,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧ����������غ㶨��,��Һ����Һ���ܽ��
��������1�����⿼�黯ѧ��������弰��д������ؼ��Ƿ��廯ѧ����������Ķ����Ƿ��ӡ�ԭ�ӡ����ӻ��ǻ��ϼۣ������ڻ�ѧ����ǰ������λ�ü����ʵ��ļ������������ر��������壬���ܸ������ʻ�ѧʽ����д������ȷ��д���ʵĻ�ѧʽ����������ȷ�Ľ�������Ŀ��
��2�����ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ɣ�
��3���ٷ����������ʵ��ܽ�����¶�Ӱ������������߶Աȣ��ж�������ʾ�����ʣ�
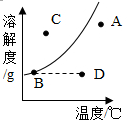
�ڸ����ܽ������ͼ�е��κ�һ�㶼��ʾ��Һ��һ���ض�״̬������A��B��C��D������ʾ��Һ��״̬��
�۶Ա�B��D�����λ�ù�ϵ���ж����ʵ���Һ����ʱ��Һ���ܶ������������ı仯��
����⣺��1������Ϊ����ǰ��������ֺ�ֻ�������壬����ʾ�������ӡ�ԭ�ӻ����ӣ�����2NH
3��ʾ�����������ӣ�
�����ӷ���ǰ��������ֱ�ʾ�������������ӣ���5Fe
2+��ʾ5���������ӣ�
��ԭ�ӷ���ǰ������ֱ�ʾ����������ԭ�ӣ���3Si��ʾ3����ԭ�ӣ�
��Ԫ�ط������Ϸ������ֱ�ʾԪ�صĻ��ϼۣ���
��ʾ+3�۵���Ԫ�أ�
��2��������ϡ���ᷴӦ�����Ȼ���������������Ӧ�Ļ�ѧ����ʽ�ǣ�Fe+2HCl�TFeCl
2+H
2����
����˿�ڴ�����ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O
2Fe
3O
4��
�۹��������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H
2O
22H
2O+O
2����
�ܸ�������ڼ��������·�Ӧ��������ء��������̺���������Ӧ�Ļ�ѧ����ʽΪ��2KMnO
4K
2MnO
4+MnO
2+O
2����
��3����A����������ƺ�D��Ķ�����̼�����ʵ��ܽ�����¶����߶���С��B���Ȼ����ܽ����Ȼ���¶����߶��������¶�Ӱ�첻��������ʾ���ܽ�����¶�Ӱ��ȴ�ܴ�
���ܽ�������ϵĵ�B��ʾ��ʱ����ҺΪ������Һ�������·��ĵ�D��A��ʾ��ʱ��ҺΪ��������Һ�������Ϸ��ĵ�C��ʾ����ҺΪ����δ�ܽ����ʵı�����Һ��
��D��Ϊ����ʱ�IJ�������Һ����ͼ��ʾ�����µ�B��ʱǡ�ñ�ɵ��µı�����Һ����������Һ����ɲ��䣬�����Һ�����������������䣻
�ʴ�Ϊ����1���������������ӣ���5���������ӣ���3����ԭ�ӣ���+3�۵���Ԫ�أ���2����Fe+2HCl�TFeCl
2+H
2������3Fe+2O
2Fe
3O
4��
��2H
2O
22H
2O+O
2������2KMnO
4K
2MnO
4+MnO
2+O
2������3����C����AD��C���۲��䣻
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף�

 ��ѧ�������������ԡ��������ԡ�ͼ�����Եȣ�
��ѧ�������������ԡ��������ԡ�ͼ�����Եȣ�

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
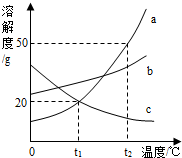 a��b��c�������ʵ��ܽ��������ͼ��ʾ������˵����ȷ���ǣ�������
a��b��c�������ʵ��ܽ��������ͼ��ʾ������˵����ȷ���ǣ�������