



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
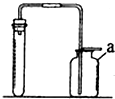 ČēĶ¼ŹĒŹµŃéŹŅÖĘČ”ĘųĢåµÄ³£¼ū×°ÖĆ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®
ČēĶ¼ŹĒŹµŃéŹŅÖĘČ”ĘųĢåµÄ³£¼ū×°ÖĆ£¬Ēė»Ų“šĻĀĮŠĪŹĢā£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢²£Į§µ¶µ¶Ķ·ĻāÉĻ½šøÕŹÆ£¬æÉŅŌÓĆĄ“²Ć²£Į§ |
| B”¢·Ą¶¾Ćę¾ßĄļĆęµÄĀĖ¶¾¹Ž¾ĶŹĒĄūÓĆ»īŠŌĢæĄ“Īü¶¾ĘųµÄ |
| C”¢¼ŅÓĆĆŗĘųŠ¹Ā©ÄÜĪŵ½Ņ»¹ÉÄŃĪŵÄĘųĢ壬ÕāŹĒCOĘųĢå |
| D”¢½ųČė¾ĆĪ“æŖĘōµÄ²Ė½ŃÖ®Ē°£¬±ŲŠė×öµĘ»šŹŌŃé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
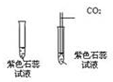 ŃéÖ¤¶žŃõ»ÆĢ¼µÄŠŌÖŹŹµŃé£ŗ
ŃéÖ¤¶žŃõ»ÆĢ¼µÄŠŌÖŹŹµŃé£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢Ģ¼ĖįøĘ CaCO3 “æ¾»Īļ |
| B”¢ĮņĖį H2SO4 Ńõ»ÆĪļ |
| C”¢ĪåŃõ»Æ¶žµŖ N2O5»ÆŗĻĪļ |
| D”¢ĀČĘų Cl2 µ„ÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
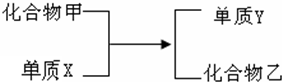 »ÆŗĻĪļ¼×Óėµ„ÖŹXŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦£¬Éś³Éµ„ÖŹY£ØŗģÉ«¹ĢĢ壩ŗĶ»ÆŗĻĪļŅŅ£ØČēĶ¼ĖłŹ¾£©£®ŌŚĻĀĮŠÉč¶ØµÄĢõ¼žĻĀ£¬ĶʶĻXæÉÄÜŹĒŹ²Ć“ĪļÖŹ£Ø¾łĢī»ÆѧŹ½£©
»ÆŗĻĪļ¼×Óėµ„ÖŹXŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦£¬Éś³Éµ„ÖŹY£ØŗģÉ«¹ĢĢ壩ŗĶ»ÆŗĻĪļŅŅ£ØČēĶ¼ĖłŹ¾£©£®ŌŚĻĀĮŠÉč¶ØµÄĢõ¼žĻĀ£¬ĶʶĻXæÉÄÜŹĒŹ²Ć“ĪļÖŹ£Ø¾łĢī»ÆѧŹ½£©²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com