
���� ��1���������������Ӵ�ʱ����������Ӧ�������ܵ����������Ӷ�����������������ʹ�������ұ�����
��2�����ʵĽṹ���������ʣ����ʵ����ʾ������ʵ���;�������������ʽ��з������ɵó���ȷ���жϣ�
��3�������������ԭ�����з�����
��� �⣺��1�����ڿ������������Ӵ���������������Ӧ��������������ѧ����ʽΪ��4Al+3O2=2Al2O3��
��2�������Ƴ���ˮ��ˮ�� ���������ĵ����ԣ������Ͻ��������Ŵ�������Ӳ��Ӳ�ȴ������Ͻ����ɻ��������������Ͻ���ܶ�С������������װƷ����������������չ�ԣ���������ѹ�� ���������ĵ����ԣ�
��3��������ʵ�������Ϳ����е�������ˮ��ͬ���õĽ����
�ʴ�Ϊ����1��4Al+3O2=2Al2O3��
��2���ڢܢݢ٢ۣ�
��3��������ˮ��
���� ������Ҫ�������������Ͻ�Ļ�ѧ���������ʷ�������ݣ����������������ʽ��У�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȣ��棩 | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�� ��g�� | NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
| Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
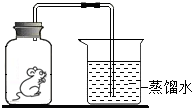 ��һֻС��������ڲ�Ϳ�г���ʯ��ˮ�ļ���ƿ�в��ܷ⣬��ͼ��ʾ������С�����Կɴ��һ��ʱ�䣬��ƿ����ģ�����ش��������⣺
��һֻС��������ڲ�Ϳ�г���ʯ��ˮ�ļ���ƿ�в��ܷ⣬��ͼ��ʾ������С�����Կɴ��һ��ʱ�䣬��ƿ����ģ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
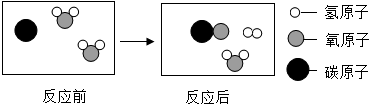
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH3 | B�� | HCl | C�� | NO2 | D�� | NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ڿ�����ȼ�շ�������������ɫ���棬����һ���д̼�����ζ������ | |
| B�� | ϸ��˿��������ȼ�ջ������䣬���ɺ�ɫ���壬�ų��������� | |
| C�� | ľ̿��������ȼ�շ��⣬����һ����ʹ����ʯ��ˮ����ǵ����� | |
| D�� | �����ڿ�����ȼ��ð�������İ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
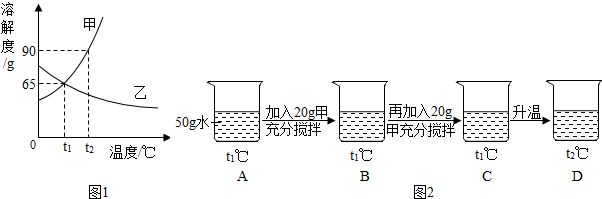
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com