
��2009?�������ھ��꼶��ѧ����ͼ��ʾװ�ÿ���ȡ�������壬��ش��������⣮
��1����a�м������������Һ��b�м���MnO
2�������ȡ��������Ӧ�Ļ�ѧ����ʽΪ
����Ӧ��MnO
2��������
������
������
��
��2����a�м������ᣬb�м���ʯ��ʯ�������ȡ������̼���壮��Ӧ�Ļ�ѧ����ʽΪ
CaCO3+2HCl�TCaCl2+H2O+CO2��
CaCO3+2HCl�TCaCl2+H2O+CO2��
��ʵ������ʯ��ʯ������Na
2CO
3�����ᷴӦ��ȡ������̼�����ԭ����Ҫ��
����������̼������̫�죬������
����������̼������̫�죬������
��
��3����a�м���ϡ���ᣬb�м���п�������ȡ��������Ӧ�Ļ�ѧ����ʽΪ
Zn+H2SO4�TZnSO4+H2��
Zn+H2SO4�TZnSO4+H2��
��ʵ���п���
��ˮ���������ſ�����
��ˮ���������ſ�����
�����ռ�������

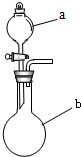 �ھ��꼶��ѧ����ͼ��ʾװ�ÿ���ȡ�������壬��ش��������⣮
�ھ��꼶��ѧ����ͼ��ʾװ�ÿ���ȡ�������壬��ش��������⣮ 2H2O+O2���������ã�
2H2O+O2���������ã�


 ��2009?�������ھ��꼶��ѧ����ͼ��ʾװ�ÿ���ȡ�������壬��ش��������⣮
��2009?�������ھ��꼶��ѧ����ͼ��ʾװ�ÿ���ȡ�������壬��ش��������⣮