
Ϋ���м���ʵʩũ�弯�й�ˮ���̣����й�ˮ�����˿��Ѵ�90%���ϡ�����ˮ��������������ɱ�������������Ȼ���������������ˮ������������Ҫ�ɷ�ΪFeS2��Ϊԭ���Ʊ��Ȼ������壨FeCl3��6H2O���Ĺ����������£�
�ش��������⣺
��1������������30%�����ᡰ���ܡ����պ�IJ�������Ҫ�ɷ��Ȼ�������д����Ӧ�ķ���ʽ_________________________________________________________��
��2�������������γ����꣬Σ���������������з�����ȥ��
����1. ������������ķ���ͨ�백ˮ�����ն�������ˮ��pH__________7����д�����ڡ�����С�ڡ����ڡ�����
����2.������������ķ���ͨ��ʯ��ʯ������Һ�У��ڿ�����������������ƺͶ�����̼���Ӷ���ȥ��������д����Ӧ�ķ���ʽ______________________________________________________��
��3������ˮ���õ�ⱥ��ʳ��ˮ�Ʊ�������Cl2����Ӧ�ķ���ʽΪ
2NaCl+2H2O 2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣�
2NaOH+ Cl2��+ H2��������Ҫ71t������������ˮ��������������Ҫ������10%�Ĵ��ζ��ٶ֣�ͬʱ�����ռ���ٶ֣�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ȡNaCl��BaCl2�Ĺ�������32��5g������100g����ˮ����ȫ�ܽ����û����Һ����μ�����������Ϊ10%��Na2SO4��Һ����Ӧ����BaSO4�������������������Na2SO4��Һ��������ϵ��ͼ��ʾ���Իش��������⣺����ʾ��BaCl2+Na2SO4�TBaSO4��+2NaCl��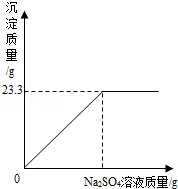
��1����ȫ��Ӧ������BaSO4���� g��
��2��ǡ����ȫ��Ӧʱ����Na2SO4��Һ�������Ƕ��ٿˣ�
��3��ǡ����ȫ��Ӧʱ������Һ�����ʵ����������Ƕ��٣�����ȷ��0.1%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
2013��3�µף��Ϻ����㽭һ������H7N9�����У����ֲ��������ڼ���Ѽ�ȷ������Ϸ��֣��Լ���Ѽ���������ʱ����15%�Ĺ���������Һ����������Ļ�ѧʽ��CH3COOOH������C2H4O3�����Լ��㣺
��1��һ������������������� ����ԭ�ӣ�
��2������������̼���⡢��Ԫ�ص��������� ����
��3������15%�Ĺ���������Һ100�ˣ������������ ���ˣ�ˮ�� ��mL������ˮ=1g/cm3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������һ����Ҫ�Ļ��ʣ������ũ����IJ���������Ҫ���ã�
��1������[CO��NH2��2]������ ������طʡ����ʡ�����
��2�����ط����У�C��H��O��N����Ԫ�ص�ԭ�Ӹ��������� ����
��3�������е�Ԫ�ص���������Ϊ�� �������������0.1%����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ľ����ũ�ҷ��ϣ�������Ҫ�ɷ���̼��أ���������أ��Ȼ��صȣ���ѧ��ȤС��Ϊ�ⶨij��ľ����Ʒ�е���Ч�ɷ֣�ȡ100��ľ�����ձ��У����ϵ���ϡ������Һ��������30gϡ����ʱ�����������ݲ�������ʱ�ձ��еIJ������������Ϊ127.8g��
̼��������ᷴӦ�Ļ�ѧ����ʽΪK2CO3+H2SO4�TK2SO4+CO2��+H2O
�������ľ�ҵ������ɷֲ�����Ԫ�أ������ᷴӦ��
�����ش�
��1��������̼��CO2�������У�̼����Ԫ�ص�ԭ�Ӹ�����Ϊ�� ��
��2��̼��أ�K2CO3������Է�������Ϊ�� ��
��3����ͼ��ʾ��Ӧ���̷ų��������������������Ĺ�ϵ���ߣ�����������غ㶨�����ͼ����������a����ֵ��a=�� ��g��
��4����ľ����Ʒ��̼��ص�������Ҫ��д��������̣�
��5��ͨ������ʵ�飬��ø�100g��ľ�������������Ϊ8.7g���Ȼ�������Ϊ2.98g����֪�طʵ���Ч�ɷ����Ȼ��ؼƣ���ò�ľ����Ʒ���Ȼ��ص���������Ϊ�� ��%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij�����������Ĵ����к�������NaCl���ʣ����Ʒ��װ���ϱ��У�̼���ơ�96%��Ϊ��֤ʵ�ò�Ʒ��̼���Ƶĺ�����ijͬѧȡ12g����Ʒ�����ձ��У��Ƶ��ձ�����Ʒ������Ϊ132.0g�ٰ�100gϡ����ƽ���ֳ�4�μ����ձ��У�ÿ�γ�ַ�Ӧ���ձ���ʣ�������������£���ÿ�η�Ӧ������CO2���嶼ȫ�����ձ����ݳ���
| ����ϡ������� | 1 | 2 | 3 | 4 |
| ����ϡ��������/g | 25 | 25 | 25 | 25 |
| ��ַ�Ӧ���ձ���ʣ����������/g | 155.2 | 178.4 | 202.6 | 227.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij��ȤС����10gþ����������������Ϊ49%��ϡ���ᷴӦ����ò���������������ϡ�����������ϵ��ͼ������þ���г����溬������þ�⣬û���������ʣ���
��1����ϡ���������Ϊ70g������������������Ϊ g��
��2���û�ѧ����ʽ˵����ϡ���������Ϊ10gʱ��Ϊʲô����������
��3������þ����þԪ�ص�����������д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����װ�ó�����ʵ������ȡ���塣���ݸ�����װ�ûش��������⣺
��1��ָ������������ƣ��� ���� ��
��2��ʵ������Dװ����ȡ�����Ļ�ѧ����ʽ��ʾΪ ������Fװ���ռ����������ʱ���� ��
��3��ʵ��ʱ��ȡ���ռ�����Ӧѡ������װ���� ,ʹ�ø���װ����ȡ�����ͻ���ŵ��� ���÷�Ӧԭ���û�ѧ����ʽ��ʾΪ ��
��4��ʵ����ѡ����ͼװ���ռ�����������Ӧ�ô� ���a����b�������롣����װ����װ��ˮʱ�����������ڲⶨ������ˮ�Ҳ���ˮ��Ӧ�������������ʱ����Ҫ �����������ƣ���������Ӧ�� ���a����b������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com