
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ��Ʒ������С�ձ��У���������ˮ����ֽ��裬���ˣ� | ||
| �� | �������ݲ��� | ���������Ѿ����ʣ� |
| ��ȡ������Һ���Թ��У�ͨ��CO2 | ��ɫ�������� | ��������û����ȫ���ʣ� �÷�Ӧ�Ļ�ѧ����ʽΪ |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ�����������Թ��У��μ�����ϡ���� | ||
| CO2+Ca��OH��2=CaCO3��+H2O |
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ�����������Թ��У��μ�����ϡ���� | ||
| CO2+Ca��OH��2=CaCO3��+H2O |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���Ͻ�ͺϳ���ά�����л��ϳɲ��� |
| B������ù���ʳ����Ⱥ������ʳ�� |
| C���ü�ȩ��Һ�������㣬�Ա��ʱ��� |
| D�����������ؽ����λ�ʧȥԭ�е��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
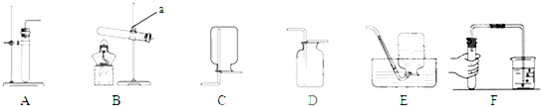
A��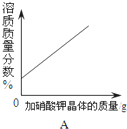 ij�¶��£���һ�������͵��������Һ�в��ϼ�������ؾ��� |
B��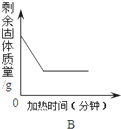 һ�����ĸ�����ؼ��ȷֽ� |
C�� ��ϡ�����еμӹ������ռ���Һ |
D�� �������ܱ����������п�������ȼ�գ�������������������ʱ��ı仯��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����Ⱥ��������Ҫ������ǯ�г� |
| B����ʣ��ҩƷ�����Ҷ���Ҫ�Ż�ԭ�Լ�ƿ |
| C���ձ����Թܶ�����ֱ�ӷ��ڻ����ϼ��� |
| D�����Թ����Һ����ȣ�Ҫ�þƾ��Ƶ����棬�Թ���б45��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
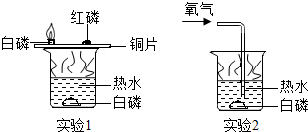
| A��ʵ��1�к���δȼ�գ�˵�������Ż����ڰ��� |
| B��ʵ��2�а���ȼ�գ�˵��ʵ��2�е���ˮ�¶ȸ���ʵ��1 |
| C��ʵ��2�����ֹͣͨ��������ȼ�ŵİ���Ϩ�� |
| D����ȼ��ȼ����Ҫ������������������ﵽ�Ż�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com