
��12�֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽���������������ʷ�����Ӧ�Ļ�ѧ����ʽΪ ���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᡣ
[ʵ��̽��]
��ȷ����Һ���Ƿ�������
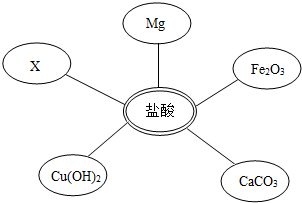
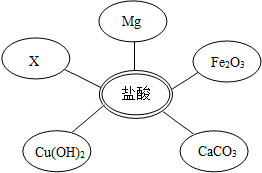
��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ��������ͼ��ʾ���������ʣ�����x�����ָʾ���е� ��Һ��

��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽 ��ȷ����Һ��һ��û�����ᡣ
(3) Y��һ�ֺ���ɫ�Ĺ����ĩ,�����ᷴӦ����Һ�ʻ�ɫ��д���������ᷴӦ�ķ���ʽ ��
��ȷ����Һ���Ƿ���Na2CO3
ijͬѧѡ�� �����Һ��pH=l0��ȷ����Һ��һ������Na2CO3��
�紦����Һ����������
���ӷ�Һ�еõ�������NaCl�����������ʵ�鷽����ơ�
|
���� |
�����Լ� |
���뷽�� |
�������� |
|
1 |
����Ca(NO3)2��Һ |
�������ᾧ |
������������ |
|
2 |
������ |
�����ᾧ |
���� |
����1�з�����Ӧ�ķ���ʽΪ ��
Na2CO3+2HCl = 2NaCl+H2O+CO2�� ʯ�� �����ݲ���(����������ޱ仯) Fe2O3 + 6HCl = 2FeCl3 + H2 O
|
���� |
�����Լ� |
���뷽�� |
�������� |
|
һ |
|
���� |
����������(����NaNO3���ɻ�����NO3��������) |
|
�� |
���� (��ϡHCl��HCl) |
|
|
Na2CO3+ Ca(NO3)2= 2NaNO3+ Ca CO3��
��������Na2CO3��Һ��ŨHCl��Ӧ����ʽΪ��Na2CO3+2HCl = 2NaCl+H2O+CO2��
�塢�š����ж���Һ������Ե�ָʾ��������ɫʯ����Һ����Ϊ�����죬���������
�ơ���Һ������ϡ���ᣬϡ�������þ��Ӧ�������塣��ˣ������ݲ����Ż�˵����ϡ���ᡣ(3)���ɫ����������������ɫ��Һ���Ȼ�����Һ����Ӧ�ķ���ʽΪ��Fe2O3 + 6HCl = 2FeCl3 + H2 O
�桢�ⶨ��Һ��PHֵ��ʵ���ҳ���PH��ֽ��
�硢������Ҫ�dz�ȥNaCl�е�Na2CO3���ӵ��Լ���̼���Ʒ�Ӧ��ͬʱ���ܲ����µ�����
|
���� |
�����Լ� |
���뷽�� |
�������� |
|
һ |
|
���� |
����������(����NaNO3���ɻ�����NO3��������) |
|
�� |
���� (��ϡHCl��HCl) |
|
|



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����
��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����| ���� | �����Լ� | ���뷽�� | �������� |
| ����Ca��NO3��2��Һ | ���ˡ������ᾧ | �����У������� ���������ʣ�����NaNO3�����ɻ�����NO�����ӣ� ���������ʣ�����NaNO3�����ɻ�����NO�����ӣ� | |
| ������ ���ᣨ��ϡHCl��HCl�� ���ᣨ��ϡHCl��HCl�� | �����ᾧ | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͬѧ����Na2CO3��Һ��Ũ�����о�����������ķ�Ӧԭ��ʱ���Է�Һ�ɷֽ���̽����
ͬѧ����Na2CO3��Һ��Ũ�����о�����������ķ�Ӧԭ��ʱ���Է�Һ�ɷֽ���̽����| ���� | �����Լ� | ���뷽�� | �������� |
| �� | ����Ca��NO3��2��Һ | ���ˡ������ᾧ | �����У������� ������������������ ������������������ |
| �� | ������ ϡ���� ϡ���� |
�����ᾧ | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?���У�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ�����̽����
��2012?���У�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ�����̽����| ���� | �����Լ� | ���뷽�� | �������� |
| һ | ������Ca��NO3��2��Һ | ���ˡ������ᾧ | ������ ������ ��ѡ����С������С�����ͬ�� |
| �� | ������ϡ���� | �����ᾧ | ���� ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | �����Լ� | ���뷽�� | �������� ���л��У���˵�����ɣ� |
| һ | ����Ca��NO3��2��Һ | ���ˡ������ᾧ | ���У����������������� ���У����������������� |
| �� | ������HCl��Һ | �����ᾧ | ���У�HCl���лӷ��� ���У�HCl���лӷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | �����Լ� | ���뷽�� | �������� ���л��У���˵�����ɣ� |
| һ | ����Ca��NO3��2��Һ | ���ˡ������ᾧ | ______ |
| �� | ������HCl��Һ | �����ᾧ | ______ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com