
�����±����ݻش����⣺
| �¶�(��) | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| NaOH�ܽ��(g/100gˮ) | 42 | 51 | 109 | 129 | 174 | 314 | 347 |
| A�ܽ��(g/100gˮ) | 0.18 | 0.17 | 0.16 | 0.14 | 0.12 | 0.095 | 0.07 |
��1��NaOH���ܽ�����¶ȱ仯�Ĺ��� ��
��2����װ��100g NaOH�����С�ձ��У�����100g��ˮ������ܽ��ⶨ��Һ�¶�Ϊ10�棬��ʱ������ҺΪ (ѡ����͡������͡�)��Һ���ٽ����ձ�����ʢ����ˮ�Ĵ��ձ��У�ʹ��Һ�¶�����60�棬��ʱ��Һ���������ܼ���������Ϊ ��
��3�������ã�2����������Һ��������ʵ�飺
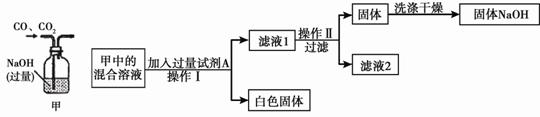
�ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ___________ __________��
����Һ1�к��е�������__________________ ![]() �� �������������__________________��
�� �������������__________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶ȣ��棩 | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| NaOH�ܽ�ȣ�g/100gˮ�� | 42 | 51 | 109 | 129 | 174 | 314 | 347 |
| A�ܽ�ȣ�g/100gˮ�� | 0.18 | 0.17 | 0.16 | 0.14 | 0.12 | 0.095 | 0.07 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����±����ݻش����⣺
| �¶�(��) | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| NaOH�ܽ��(g/100gˮ) | 42 | 51 | 109 | 129 | 174 | 314 | 347 |
| A�ܽ��(g/100gˮ) | 0.18 | 0.17 | 0.16 | 0.14 | 0.12 | 0.095 | 0.07 |
��1��NaOH���ܽ�����¶ȱ仯�Ĺ��� ��
��2����װ��100g NaOH�����С�ձ��У�����100g��ˮ������ܽ��ⶨ��Һ�¶�Ϊ10�棬��ʱ������ҺΪ (ѡ����͡������͡�)��Һ���ٽ����ձ�����ʢ����ˮ�Ĵ��ձ��У�ʹ��Һ�¶�����60�棬��ʱ��Һ���������ܼ���������Ϊ ��
��3�� �����ã�2����������Һ��������ʵ�飺
 �ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________��
�ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________��
����Һ1�к��е�������__________________�� �������������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�(��) | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| NaOH�ܽ��(g/100gˮ) | 42 | 51 | 109 | 129 | 174 | 314 | 347 |
| A�ܽ��(g/100gˮ) | 0.18 | 0.17 | 0.16 | 0.14 | 0.12 | 0.095 | 0.07 |
 �ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________��
�ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�챱���г������п�һģ��ѧ�Ծ� ���������� ���ͣ������
�����±����ݻش����⣺
| �¶�(��) | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| NaOH�ܽ��(g/100gˮ) | 42 | 51 | 109 | 129 | 174 | 314 | 347 |
| A�ܽ��(g/100gˮ) | 0.18 | 0.17 | 0.16 | 0.14 | 0.12 | 0.095 | 0.07 |
 �ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________��
�ټ����Լ�Aʱ������Ӧ�Ļ�ѧ����ʽΪ__________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȣ��棩 | 0 | 10 | 20 | 40 | 60 | 80 | 100 |
| NaOH�ܽ�ȣ�g/100gˮ�� | 42 | 51 | 109 | 129 | 174 | 314 | 347 |
| A�ܽ�ȣ�g/100gˮ�� | 0.18 | 0.17 | 0.16 | 0.14 | 0.12 | 0.095 | 0.07 |

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com