
| µ—È≤Ω÷˺∞≤Ÿ◊˜ | µ—Èœ÷œÛ | µ—ÈΩ·¬€ |
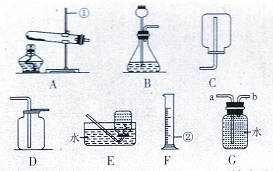
| »°—˘”⁄ ‘πÐ÷–£¨º”»Î◊„¡ø’Ù¡ÛÀÆ’Òµ¥£¨æ≤÷√ ¢Ÿ»°…œ≤„«Â“∫£¨µŒ»ÎŒÞ…´∑”Ù ‘“∫ ¢⁄µπ»•…œ≤„«Â“∫£¨‘ŸœÚ ‘πÐ÷–◊¢»Îœ°—ŒÀ· | ¢ŸŒÞ…´∑”Ù ‘“∫±‰∫Ï ¢⁄ | ≤ø∑÷±‰÷ |
| ¢ŸŒÞ…´∑”Ù ‘“∫≤ª±‰∫Ï ¢⁄ | | |
| ¢Ÿ ¢⁄√ª”–∆¯≈ð≤˙…˙ | |
| µ—È≤Ω÷˺∞≤Ÿ◊˜ | µ—Èœ÷œÛ | µ—ÈΩ·¬€ |
| | ¢Ÿ ¢⁄”–∆¯≈ð≤˙…˙ | |
| ¢Ÿ ¢⁄”–∆¯≈ð≤˙…˙ | »´≤ø±‰÷ | |
| ¢ŸŒÞ…´∑”Ù ‘“∫±‰∫Ï ¢⁄ | √ª”–±‰÷ |



| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ

≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
| µ—È≤Ω÷Ë | µ—Èœ÷œÛ | µ—ÈΩ·¬€ |
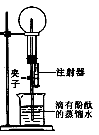
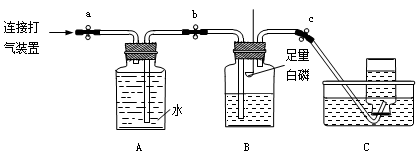
| 1°¢Ω´∆§µ∞¡œƒýµƒ‘≠¡œ∑≈»Î…’±≠÷–£¨º” ¡øµƒÀÆΩ¡∞Ë°¢≥‰∑÷∑¥”¶ | ‘πÐÕ‚±⁄ £¨”–∞◊…´≥¡µÌ…˙≥… | ¬À‘¸÷–“ª∂®”– °£ |
| 2°¢Ω´…œ ˆ…’±≠÷–µƒŒÔ÷ π˝¬À°¢œ¥µ”£¨¬À‘¸÷–µŒº”œ°—ŒÀ· | ”–∆¯≈ð√∞≥ˆ | |
| 3°¢»°…Ÿ¡ø¬À‘¸”⁄ ‘πÐ÷–º”ÀÆ≥‰∑÷»ÐΩ‚£¨»°…œ≤„«Â“∫µŒº” | …œ≤„«Â“∫”…ŒÞ…´±‰≥…∫Ï…´ | |
| 4°¢»°¬À“∫»˝∑ð£¨∑÷±µŒº”∑”Ù°¢œ°—ŒÀ·∫ÕÕ®»ÎCO2∆¯Ã | »Ð“∫±‰∫Ï…´°¢ŒÞ√˜œ‘œ÷œÛ°¢”–∞◊…´ªÎ◊« | ¬À“∫÷–µƒ»Ð÷ “ª∂®”– |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
| µ—È≤Ω÷Ë | µ—Èœ÷œÛ | µ—ÈΩ·¬€ |
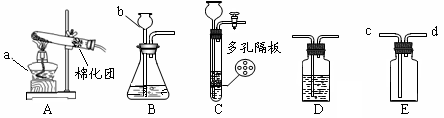
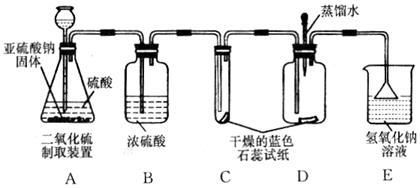
| »°—˘£¨º” ¡øÀÆ£¨Ω¡∞Ë£¨π˝¬À ¢Ÿ»°…Ÿ¡ø¬À“∫”⁄ ‘πÐ÷–£¨µŒ»Î∑”Ù ‘“∫ ¢⁄»°…Ÿ¡ø¬À‘¸”⁄ ‘πÐ÷–£¨º”»Î—ŒÀ· | ¢Ÿ¬À“∫≤ª±‰…´ ¢⁄”–∆¯≈ð≤˙…˙ | «‚—ıªØ∏∆»´≤ø±‰Œ™ÃºÀ·∏∆ |
| µ—È≤Ω÷Ë | µ—Èœ÷œÛ | µ—ÈΩ·¬€ |
| »°—˘£¨º” ¡øÀÆ£¨Ω¡∞Ë£¨π˝¬À ¢Ÿ»°…Ÿ¡ø¬À“∫”⁄ ‘πÐ÷–£¨µŒ»Î∑”Ù ‘“∫ ¢⁄»°…Ÿ¡ø¬À‘¸”⁄ ‘πÐ÷–£¨º”»Î—ŒÀ· | ¢Ÿ | |
| ¢⁄ |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
| ŒÔ ÷ | MgO | MgCl2 | Mg3N2 | Mg(NO3)2 | MgCO3 | Mg(OH)2 |
| —’ …´ | ∞◊…´ | ∞◊…´ | µ≠ª∆…´ | ∞◊…´ | ∞◊…´ | ∞◊…´ |
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫≥ı÷–ªØ—ß ¿¥‘¥£∫≤ªœÍ –գ∫ µ—ÈÂ
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
∞Ÿ∂»÷¬–≈ - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com