
ijĶ¬Ń§ŌŚŃ§Ļ°ČÜŅŗµÄĖį¼īŠŌŹ±·¢ĻÖŃĪČÜŅŗNaCl³ŹÖŠŠŌNa2CO3ČÜŅŗ³Ź¼īŠŌ£¬øĆĶ¬Ń§ČĻĪŖÓŠæÉÄÜÓŠµÄŃĪČÜŅŗ»į³ŹĖįŠŌ£¬ÉĻĶų²é׏ĮĻ·¢ĻÖ£ØNH4£©2SO4£¬FeCl3ČÜŅŗ³ŹĖįŠŌ£®øĆĶ¬Ń§Ą“ŠĖȤĮĖŠ“³öČēĻĀµÄѧĻ°Š”½į£¬ĒėÄć×öŅ»×ö£ŗ
£Ø1£©ŃĪČÜŅŗpHµÄæÉÄÜĪŖ£Ø½«ÄćµÄ¼ŁÉčĢīČėæÕøńÖŠ£©¢Ł”” ””¢Ś”” ””¢ŪpH=7
£Ø2£©ŅŖ²ā¶ØøĆČÜŅŗµÄĖį¼īŠŌÓĆ”” ””£¬ČēŅŖÖŖµĄøĆČÜŅŗµÄĖį¼ī¶ČæÉÓĆ”” ””£®²Ł×÷·½·Ø”” ””£®
£Ø3£©”°×ƼŚŅ»Ö¦»Ø£¬Č«ææ·Źµ±¼Ņ£®”±£ØNH4£©2SO4ŹĒŅ»ÖÖµŖ·ŹÄÜ“Ł½ųÅ©×÷ĪļµÄ¾„”¢Ņ¶Éś³¤ĆÆŹ¢£¬ÄÜŹ¹×ĻÉ«ŹÆČļŹŌŅŗ±ä”” ””É«£®
£Ø4£©ļ§Ģ¬µŖ·ŹµÄ¼ģ²ā·½·ØŹĒ£ŗ”” ””£®
£Ø5£©øĆĶ¬Ń§¼ÓČČFeCl3ČÜŅŗ·¢ĻÖÓŠŗģŗÖÉ«³ĮµķÉś³É£¬ĒėŠ“³ö»Æѧ·½³ĢŹ½£ŗ”” ””£®£ØĢįŹ¾£ŗCu£ØOH£©2µČÄŃČÜŠŌ¼īŌŚpH£¾5ŃĪĖįÖŠ²»Čܽā£©
£Ø6£©ĪŖŹ²Ć“Na2CO3ČÜŅŗ³Ź¼īŠŌ£¬£ØNH4£©2SO4£¬FeCl3ČÜŅŗ³ŹĖįŠŌ£¬ÉĻĶų²é²»ŹĒŗÜĆ÷°×£¬ŹĒøßÖŠŅŖѧµÄ£¬µČÉĻøßÖŠŗóĪŅŅ»¶ØŅŖøćĒ峞£®
£Ø1£©pH£¾7£¬pH£¼7
£Ø2£©Ėį¼īÖøŹ¾¼Į£¬pHŹŌÖ½£¬ÓĆ²£Į§°ōÕŗČ”“ż²āŅŗĶæŌŚpHŹŌÖ½ÉĻ£¬°ŃĻŌŹ¾µÄŃÕÉ«Óė±ź×¼±ČÉ«æضŌÕÕ¼“æÉ
£Ø3£©ŗģ £Ø4£©ÓėŹģŹÆ»Ņ»ģŗĻŃŠÄ„£¬¹Ū²ģŹĒ·ńÓŠ“Ģ¼¤ŠŌµÄĘųĪ¶²śÉś
£Ø5£©FeCl3+3H20 Fe£ØOH£©3”ż+3HCl
Fe£ØOH£©3”ż+3HCl
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©pH£¾7µÄČÜŅŗ³Ź¼īŠŌ£¬pH£¼7µÄČÜŅŗ³ŹĖįŠŌ£¬pH=7µÄČÜŅŗ³ŹÖŠŠŌ£¬ŃĪČÜŅŗæÉÄܳŹ¼īŠŌ”¢ĖįŠŌ»ņÖŠŠŌ£¬¹ŹĢī£ŗpH£¾7£¬pH£¼7£»
£Ø2£©Ėį¼īÖøŹ¾¼ĮæÉŅŌ²ā¶ØČÜŅŗµÄĖį¼īŠŌ£¬²ā¶ØČÜŅŗµÄĖį¼ī¶ČŹ¹ÓƵďĒpHŹŌÖ½£¬²ā¶ØŹ±ŅŖÓĆ²£Į§°ōÕŗČ”“ż²āŅŗĶæŌŚpHŹŌÖ½ÉĻ£¬°ŃĻŌŹ¾µÄŃÕÉ«Óė±ź×¼±ČÉ«æضŌÕÕ¼“æÉ£¬¹ŹĢī£ŗĖį¼īÖøŹ¾¼Į£¬pHŹŌÖ½£¬ÓĆ²£Į§°ōÕŗČ”“ż²āŅŗĶæŌŚpHŹŌÖ½ÉĻ£¬°ŃĻŌŹ¾µÄŃÕÉ«Óė±ź×¼±ČÉ«æضŌÕÕ¼“æÉ£»
£Ø3£©£ØNH4£©2SO4ČÜŅŗ³ŹĖįŠŌ£¬ĖįŠŌČÜŅŗÄÜŹ¹ŹÆČļŹŌŅŗ±äŗģ£¬¹ŹĢī£ŗŗģ£»
£Ø4£©ļ§Ģ¬µŖ·ŹÓė¼īŠŌĪļÖŹ»ģŗĻ»į²śÉśÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄ°±Ęų£¬¹ŹæÉŅŌŹ¹ÓĆÓėŹģŹÆ»Ņ»ģŗĻŃŠÄ„µÄ·½·Ø½ųŠŠ¼ģŃéļ§Ģ¬µŖ·Ź£¬¹ŹĢī£ŗÓėŹģŹÆ»Ņ»ģŗĻŃŠÄ„£¬¹Ū²ģŹĒ·ńÓŠ“Ģ¼¤ŠŌµÄĘųĪ¶²śÉś£»
£Ø5£©¼ÓČČĀČ»ÆĢśČÜŅŗÉś³ÉŗģŗÖÉ«³Įµķ£¬ÕāŹĒĀČ»ÆĢśÓėĖ®·“Ӧɜ³ÉĮĖĒāŃõ»ÆĢśŗĶŃĪĖįµÄŌµ¹Ź£¬øł¾ŻĢāøÉĢį¹©µÄŠÅĻ¢æÉŅŌÖŖµĄ£¬ŌŚpH£¾5µÄŃĪĖįČÜŅŗÖŠÄŃČÜŠŌ¼ī²»Čܽā£¬¹ŹĢī£ŗFeCl3+3H20 Fe£ØOH£©3”ż+3HCl£®
Fe£ØOH£©3”ż+3HCl£®
æ¼µć£ŗŃĪµÄ»ÆѧŠŌÖŹ£»ČÜŅŗµÄĖį¼ī¶Č²ā¶Ø£»ČÜŅŗµÄĖį¼īŠŌ²ā¶Ø£»³£¼ū»Æ·ŹµÄÖÖĄąŗĶ×÷ÓĆ£»ļ§Ģ¬µŖ·ŹµÄ¼ģŃ飻ŹéŠ“»Æѧ·½³ĢŹ½”¢ĪÄ×Ö±ķ“ļŹ½”¢µēĄė·½³ĢŹ½£®
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖ³£¼ūŃĪµÄŠŌÖŹŅŌ¼°ÓŠ¹ŲČÜŅŗĖį¼īŠŌµÄ²ā¶Ø£¬Ķź³É“ĖĢā£¬æÉŅŌŅĄ¾ŻŅŃÓŠµÄÖŖŹ¶½ųŠŠ


 ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
ĆūŹ¦µć¾¦×Ö“Ź¾ä¶ĪĘŖĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÄæĒ°Å©“åÕżŌŚĶĘ¹ć”°²āĶĮÅä·½Ź©·Ź”±¼¼Źõ£¬Å©¼¼Ō±¶ŌijĶĮµŲ¼ģ²āŗóøų³öĮĖŹ©·ŹÅä·½£¬Åä·½ÖŠÖ÷ŅŖÓŠKNO3”¢K2SO4”¢NH4NO3”¢NH4HCO3µČĪļÖŹ£¬ÉĻŹö·ŹĮĻÖŠŹōÓŚø“ŗĻ·ŹĮĻµÄŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
Š”³ĒĶ¬Ń§½«Ņ»øöŠĀĻŹµÄ¼¦µ°·ÅŌŚŹ¢ÓŠ×ćĮæĻ”ŃĪĖįµÄ²£Į§±
ÖŠ£¬æɹŪ²ģµ½µÄĻÖĻóŹĒ£Ø1£©___________________________________£»
²śÉś“ĖĻÖĻóµÄŌŅņŹĒ£Ø2£©______________________________________
____________________________________________________________
___________________________________________________________ӣ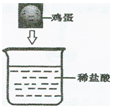
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ČēĶ¼ŹĒijĶ¬Ń§¼ų±šĢ¼ĖįĒāļ§”¢ĮņĖįļ§”¢ĻõĖįļ§ČżÖֻƷŹµÄ¹ż³Ģ£Ø·“Ó¦Ģõ¼žĪ“±ź³ö£©£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©²½Öč¢ŁÖŠĶعż¹Ū²ģµ½”” ””ĻÖĻó£¬æÉŅŌ¼ų±š³öĢ¼ĖįĒāļ§£®
£Ø2£©²½Öč¢ŚÖŠĖłŠčŹŌ¼ĮæÉŅŌŃ”ÓĆĮ½ÖÖ²»Ķ¬Ąą±š£Ø°“Ėį”¢¼ī”¢ŃĪ”¢Ńõ»ÆĪļ½ųŠŠ·ÖĄą£©µÄĪļÖŹ£¬Ęä»ÆѧŹ½·Ö±šĪŖ”” ”””¢”” ””£®
ĒėŠ“³öĮņĖįļ§·Ö±šÓėÕāĮ½ÖÖŹŌ¼Į·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ”” ”””¢”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ÉśŹÆ»ŅæÉ×÷Ź³Ę·°ü×°“üÄŚµÄøÉŌļ¼Į£¬ĘäøÉŌļµÄŌĄķŹĒŹ²Ć“£ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£© ÓĆ¹żŅ»¶ĪŹ±¼äŗó£¬øÉŌļ¼ĮÖŠµÄĪļÖŹ×ī¶ąÓŠ ÖÖ£®ÓŠŠ©Ź³Ę·°ü×°“üÄŚµÄøÉŌļ¼ĮŹĒĢś·Ū£¬Ģś·ŪŹ§Š§ŗó»į±ä³É É«£»Ģś·ŪĪŖŹ²Ć“±ČÉśŹÆ»ŅøüÄÜŃÓ³¤Ź³Ę·±£ÖŹĘŚ£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
Ķ¼ŹĒij»Æ¹¤³§Éś²śÉÕ¼īµÄ¹¤ŅµĮ÷³ĢĶ¼£®
Ēėøł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĒėŠ“³öXĪļÖŹŌŚŹµŃéŹŅÖŠµÄŅ»ÖÖÓĆĶ¾”” ””£®
£Ø2£©·“Ó¦³ŲÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”” ””£®
£Ø3£©²Ł×÷¢ŁµÄĆū³ĘŹĒ”” ””£¬½į¾§µĆµ½µÄ¹ĢĢåÉÕ¼īÖŠæÉÄÜŗ¬ÓŠÉŁĮæµÄ”” ””£ØŠ“»ÆѧŹ½£©£®
£Ø4£©ĀĖŅŗDæɼÓČė·“Ó¦³ŲŃ»·ŌŁĄūÓĆ£¬ÄæµÄŹĒ½µµĶÉś²ś³É±¾ŗĶ·ĄÖ¹”” ””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢ½¾æĢā
(4·Ö)ŹµŃéŹŅÓŠŅ»Ęæ±źĒ©ĘĘĖšµÄČÜŅŗ£¬ŅŃÖŖĘäÖŠŗ¬ÓŠÄĘŌŖĖŲ£¬ĶĘ²āæÉÄÜŹĒNa2SO4”¢Na2CO3”¢NaCl”¢NaOHĘäÖŠµÄŅ»ÖÖ»ņ¼øÖÖ£¬ĪŖĢ½¾æĘä³É·Ö£¬×öĮĖŅŌĻĀŹµŃé£ŗ
¢Ł²āµĆČÜŅŗpH“óÓŚ7£»
¢Ś¼ÓČė×ćĮæBaCl2ČÜŅŗŗó£¬ÓŠ°×É«³Įµķ²śÉś£¬¹żĀĖ£»
¢ŪĻņĀĖ³öµÄ³ĮµķÖŠµĪ¼ÓĻ”ĻõĖį£¬²æ·Ö³ĮµķČܽāĒŅÓŠĘųĢå²śÉś£»
ĒėĶĘ²ā£ŗ£Ø1£©øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠŹ²Ć“ĪļÖŹ£æŠ“³öÉś³É¢ŪÖŠĪ“Čܽā³ĮµķµÄ»Æѧ·½³ĢŹ½”£
£Ø2£©ÓūÖ¤Ć÷ČÜŅŗÖŠ²»ŗ¬NaOH£¬ŠčŅŖ²¹³äŹ²Ć“ŹµŃé£æĒėŠ“³öŹµŃé²½Öč¼°ĻÖĻó”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢ½¾æĢā
£Ø7·Ö£©ŅŃÖŖ£ŗ³£ĪĀĻĀ£¬CO2”¢Ė®¶¼ÄÜÓė¹żŃõ»ÆÄĘ£Ø»ÆѧŹ½ Na2O2£©·“Ó¦²śÉś O2£¬ĘäÖŠCO2 Óė Na2O2 ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ 2CO2 + 2Na2O2 =2Na2CO3 +O2”£Ä³Š£»Æѧ»ī¶ÆŠ”×éĪŖĢ½¾æ CO2ÓėNa2O2·“Ó¦µÄ²śĪļµÄŠŌÖŹ£¬Éč¼ĘĮĖČēĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ”£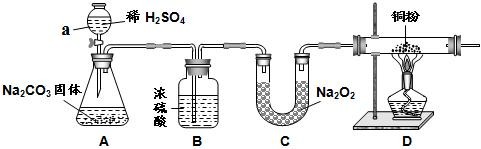
¢ÅŅĒĘ÷ a µÄĆū³ĘŹĒ£ŗ ”£
¢Ę×°ÖĆ B µÄ×÷ÓĆŹĒ ”£
¢Ē×°ÖĆ A ÖŠ£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
¢Č×°ÖĆ D ²£Į§¹ÜÖŠ£¬æÉŅŌ¹Ū²ģµ½µÄĻÖĻóŹĒ £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢É·“Ó¦ŗ󣬽«×°ÖĆ C ÖŠµÄ¹ĢĢåĪļÖŹČÜÓŚĖ®Åä³ÉČÜŅŗ£¬Č»ŗóĻņøĆČÜŅŗÖŠ¼ÓČė £ØŃ”Ģī”°ŃĪĖį”±”¢”°CaCl2 ČÜŅŗ”±”¢”°ŹÆČļŹŌŅŗ”±Ö®Ņ»£©£¬»į³öĻÖ µÄĻÖĻó”£
¢Ź·“Ó¦Ķź±Ļŗ󣬲āµĆ×°ÖĆ C µÄ×ÜÖŹĮæŌö¼ÓĮĖ 14g£¬Ōņ²śÉś O2 µÄÖŹĮæĪŖ g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢ½¾æĢā
ŹµŃéŹŅÓŠŅ»Ęæ±£¹Ü²»µ±µÄŹŌ¼Į£ØČēĶ¼£©£¬Ęä²ŠČ±µÄ±źĒ©ÖŠÖ»Ź£ĻĀ”°Na”±ŗĶ”°10%”±×ÖŃł”£ŅŃÖŖĖüŹĒĪŽÉ«ŅŗĢ壬ŹĒ³õÖŠ»Æѧ³£ÓƵďŌ¼Į”£
£Ø1£©øł¾ŻŹÜĖš±źĒ©µÄĒéæöÅŠ¶Ļ£¬ÕāĘæŹŌ¼Į²»æÉÄÜŹĒ
A£®Ėį B£®¼ī C£®ŃĪ
£Ø2£© ŅŃÖŖ ¢ń£®³õÖŠ»Æѧ³£¼ūµÄŗ¬ÄĘ»ÆŗĻĪļÓŠNaCl”¢NaOH”¢Na2CO3”¢NaHCO3”£
¢ņ£®Na2CO3ŗĶNaHCO3ČÜŅŗ¶¼³Ź¼īŠŌ”£
¢ó£®ŹŅĪĀ£Ø20”ę£©Ź±£¬ĖÄÖÖĪļÖŹµÄČܽā¶ČµÄŹż¾ŻČēĻĀĶ¼£ŗ
| ĪļÖŹ | NaCl | NaOH | Na2CO3 | NaHCO3 |
| Čܽā¶Čg | 36 | 109 | 215 | 9£®6 |
| ²Ł×÷²½Öč | ŹµŃéĻÖĻó | ½įĀŪ¼°»Æѧ·½³ĢŹ½ |
| ȔѳӌŹŌ¹ÜÖŠ£¬µĪ¼Ó | ²śÉś“óĮæµÄĘųÅŻ | øĆČÜŅŗŹĒ £¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com