
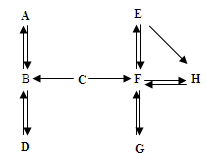
£Ø7·Ö£©A~HŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ”£Ēė½įŗĻĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ØĶ¼ÖŠ”°”ś”±±ķŹ¾ĪļÖŹ¼ä“ęŌŚ×Ŗ»Æ¹ŲĻµ£©”£
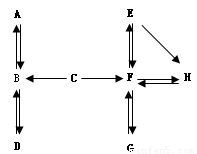
£Ø1£©AŌŚ±ź×¼×“æöĻĀŹĒĆܶČ×īŠ”µÄĘųĢ壬AµÄ»ÆѧŹ½ŹĒ_____£»A”¢B”¢CÖŠŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬C”śBČÜŅŗ³Ź»ĘÉ«£¬ÓÉ“ĖĶʶĻÓėC·“Ӧɜ³ÉBµÄĪļÖŹŹĒ_____”£
£Ø2£©DĪŖĘųĢåµ„ÖŹ£¬Š“³öB”śDµÄ»Æѧ·½³ĢŹ½_____”£
£Ø3£©¹¤Ņµ³£ÓĆG”śF·“Ó¦Į¶Ģś£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____”£
£Ø4£©E”śH²śÉś°×É«³Įµķ£¬¹żĀĖŗóĻņĀĖŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬²śÉśĘųĢåĢå»żÓėĖł¼ÓĻ”ŃĪĖįĢå»żµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£ĀĖŅŗÖŠµÄČÜÖŹŹĒ_____£»Š“³öE”śHµÄ»Æѧ·½³ĢŹ½_____£»F”śEµÄ»Æѧ·½³ĢŹ½_____”£
£Ø1£©H2 £»Fe2O3
£Ø2£©2H2O 2H2”ü+O2”ü
2H2ӟ+O2ӟ
£Ø3£©3CO + Fe2O3 2Fe + 3CO2
2Fe + 3CO2
£Ø4£©NaOH”¢Na2CO3
Na2CO3 + Ca(OH)2 CaCO3”ż+ 2NaOH
2 NaOH + CO2 Na2CO3+ H2O
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©AŌŚ±ź×¼×“æöĻĀŹĒĆܶČ×īŠ”µÄĘųĢ壬AŹĒĒāĘų£¬AµÄ»ÆѧŹ½ŹĒH2 £»C”śBČÜŅŗ³Ź»ĘÉ«£¬ĖµĆ÷ČÜŅŗÖŠŗ¬ÓŠĢśĄė×Ó£¬ŌŁøł¾Ż”°A”¢B”¢CÖŠŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ”±£¬ŌņBĪŖĖ®£¬CÖŠŗ¬ÓŠĒāŌŖĖŲ£¬ĒŅÄÜÓėŗ¬ĢśµÄ»ÆŗĻĪļ·“Ó¦£¬ĖłŅŌCĪŖŃĪĖį£»DĪŖĘųĢåµ„ÖŹ£¬BĪŖĖ®£¬æÉĶĘ³öDĪŖŃõĘų£»ÓɲśÉśĘųĢåµÄĶ¼ĻóæÉÖŖ£¬ČÜŅŗÖŠ±Ų¶ØĪŖæÉČÜŠŌ¼īŗĶĢ¼ĖįŃĪµÄ»ģŗĻĪļ£¬ĒŅøĆĢ¼ĖįŃĪÓėøĆ¼ī²»·“Ó¦£¬ĖłŅŌČÜÖŹĪŖĒāŃõ»ÆÄĘÓėĢ¼ĖįÄĘ£¬ŌņHĪŖĢ¼ĖįøĘ£¬ÓÉÓŚ¹¤Ņµ³£ÓĆG”śF·“Ó¦Į¶Ģś£¬ÓÖŅņĪŖGFæÉŅŌĻą»„×Ŗ»Æ£¬ŌņFĪŖ¶žŃõ»ÆĢ¼£¬GĪŖŅ»Ńõ»ÆĢ¼”£
£Ø2£©B”śDµÄ»Æѧ·½³ĢŹ½ĪŖ2H2O 2H2”ü+O2”ü”£
2H2ӟ+O2ӟӣ
£Ø3£©¹¤ŅµÓĆG”śF·“Ó¦Į¶Ģś£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ3CO + Fe2O3 2Fe + 3CO2”£
2Fe + 3CO2ӣ
£Ø4£©ÓɲśÉśĘųĢåµÄĶ¼ĻóæÉÖŖ£¬ĀĖŅŗÖŠČÜÖŹĪŖĒāŃõ»ÆÄĘÓėĢ¼ĖįÄĘ£¬E”śHŹĒĢ¼ĖįÄĘÓėĒāŃõ»ÆøĘ·“Ó¦£¬»Æѧ·½³ĢŹ½ĪŖNa2CO3 + Ca(OH)2 CaCO3”ż+ 2NaOH£¬
F”śEµÄ»Æѧ·½³ĢŹ½ĪŖ2 NaOH + CO2 Na2CO3+ H2O”£
æ¼µć£ŗĪļÖŹĶʶĻ”£
µćĘĄ£ŗĶʶĻĪļÖŹ£¬ŅŖøł¾ŻĢāÖŠŠÅĻ¢£¬Ń°ÕŅĶ»ĘĘæŚ£¬²¢ĒŅŅŖÉĘÓŚÕ¹æŖŗĻĄķµÄĮŖĻė£¬±¾ĢāÄŃ¶Č½Ļ“ó”£


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 Éś»īÖŠ“¦“¦ÓŠ»Æѧ£®ČĻŹ¶ŗĶĢ½¾æÉķ±ßµÄ»ÆѧĪļÖŹ£®ŹĒѧĻ°»ÆѧµÄÖŲŅŖÄŚČŻ£ŗĒėÓĆĖłŃ§µÄ»ÆѧÖŖŹ¶ĢīæÕ£®
Éś»īÖŠ“¦“¦ÓŠ»Æѧ£®ČĻŹ¶ŗĶĢ½¾æÉķ±ßµÄ»ÆѧĪļÖŹ£®ŹĒѧĻ°»ÆѧµÄÖŲŅŖÄŚČŻ£ŗĒėÓĆĖłŃ§µÄ»ÆѧÖŖŹ¶ĢīæÕ£®
| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø7·Ö£©A~HŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ”£Ēė½įŗĻĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ØĶ¼ÖŠ”°”ś”±±ķŹ¾ĪļÖŹ¼ä“ęŌŚ×Ŗ»Æ¹ŲĻµ£©”£
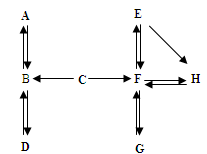
1.AŌŚ±ź×¼×“æöĻĀŹĒĆܶČ×īŠ”µÄĘųĢ壬AµÄ»ÆѧŹ½ŹĒ £»A”¢B”¢CÖŠŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬C”śBČÜŅŗ³Ź»ĘÉ«£¬ÓÉ“ĖĶʶĻÓėC·“Ӧɜ³ÉBµÄĪļÖŹŹĒ
2.DĪŖĘųĢåµ„ÖŹ£¬Š“³öB”śDµÄ»Æѧ·½³ĢŹ½ ”£
3.¹¤Ņµ³£ÓĆG”śF·“Ó¦Į¶Ģś£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
4.E”śH²śÉś°×É«³Įµķ£¬¹żĀĖŗóĻņĀĖŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬²śÉśĘųĢåĢå»żÓėĖł¼ÓĻ”ŃĪĖįĢå»żµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£
ĀĖŅŗÖŠµÄČÜÖŹŹĒ £»Š“³öE”śHµÄ»Æѧ·½³ĢŹ½ £»
F”śEµÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012ÄźĮ¬ŌĘøŪŹŠÖŠæ¼Ä£ÄāŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĶʶĻĢā
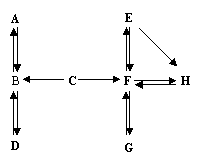
£Ø7·Ö£©A~HŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ”£Ēė½įŗĻĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ØĶ¼ÖŠ”°”ś”±±ķŹ¾ĪļÖŹ¼ä“ęŌŚ×Ŗ»Æ¹ŲĻµ£©”£

1.AŌŚ±ź×¼×“æöĻĀŹĒĆܶČ×īŠ”µÄĘųĢ壬AµÄ»ÆѧŹ½ŹĒ £»A”¢B”¢CÖŠŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬C”śBČÜŅŗ³Ź»ĘÉ«£¬ÓÉ“ĖĶʶĻÓėC·“Ӧɜ³ÉBµÄĪļÖŹŹĒ
2.DĪŖĘųĢåµ„ÖŹ£¬Š“³öB”śDµÄ»Æѧ·½³ĢŹ½ ”£
3.¹¤Ņµ³£ÓĆG”śF·“Ó¦Į¶Ģś£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
4.E”śH²śÉś°×É«³Įµķ£¬¹żĀĖŗóĻņĀĖŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬²śÉśĘųĢåĢå»żÓėĖł¼ÓĻ”ŃĪĖįĢå»żµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£

ĀĖŅŗÖŠµÄČÜÖŹŹĒ £»Š“³öE”śHµÄ»Æѧ·½³ĢŹ½ £»
F”śEµÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

|
 |
£Ø1£©AŌŚ±ź×¼×“æöĻĀŹĒĆܶČ×īŠ”µÄĘųĢ壬AµÄ»ÆѧŹ½ŹĒ_____£»A”¢B”¢CÖŠŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬C”śBČÜŅŗ³Ź»ĘÉ«£¬ÓÉ“ĖĶʶĻÓėC·“Ӧɜ³ÉBµÄĪļÖŹŹĒ_____”£
£Ø2£©DĪŖĘųĢåµ„ÖŹ£¬Š“³öB”śDµÄ»Æѧ·½³ĢŹ½_____”£
£Ø3£©¹¤Ņµ³£ÓĆG”śF·“Ó¦Į¶Ģś£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_____”£
£Ø4£©E”śH²śÉś°×É«³Įµķ£¬¹żĀĖŗóĻņĀĖŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬
²śÉśĘųĢåĢå»żÓėĖł¼ÓĻ”ŃĪĖįĢå»żµÄ¹ŲĻµČēÓŅĶ¼ĖłŹ¾”£
ĀĖŅŗÖŠµÄČÜÖŹŹĒ_____£»
Š“³öE”śHµÄ»Æѧ·½³ĢŹ½_____£»
F”śEµÄ»Æѧ·½³ĢŹ½_____”£
 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com