

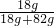 ��100%=18%��
��100%=18%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�γ��е�һ������ѧ���꼶�п�����ģ�⿼�Ի�ѧ�Ծ� ���������� ���ͣ������
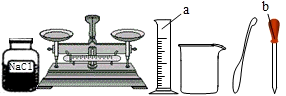
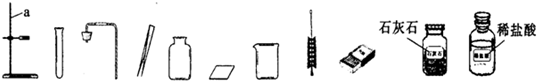
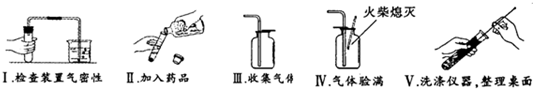
��16�֣������п���ѧʵ������������������⣺����������ȡ�ڶ�����̼����ȡ������50g5%��NaCl��Һ����ͼ�Ļ�ѧ���ʢݴ��ε��ᴿ��̽��ij���ε����ʡ����Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��
��ش�

(1)ָ����ͼ������a�����ƣ� �� д������ͼҩƷ��������ʵ��ʱ������Ӧ�Ļ�ѧ����ʽ��
(2)��ʵ��̨���ṩ��������ҩƷ������ΪС���鵽���ǵ� �����⣻
(3)������С����ɸ�ʵ�鲿�ֲ������̵�ʾ��ͼ��û��ָ���IJ�������ȷ�������ֱ���ÿ�������ȷ��1�֣�����10�֣�ʵ����Ϻ�С������8�֡����ҳ���ʧ�ֵIJ�����˵��ԭ�� �� ��
(4)������������(ҩƷ��ѡ)��Ҳ�������һ�ֳ��������ʵ������ȡ����ѧ����ʽΪ�� �����������ܺ������� (��һ�ֲ�����������)������װ�ɸ�������������ķ���װ�á�
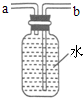
��5������ͼ��ʾװ���ռ�����������Ӧ�� ���a����b�����˵��롣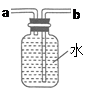
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�γ��о��꼶�п�����ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ������
��16�֣������п���ѧʵ������������������⣺����������ȡ�ڶ�����̼����ȡ������50g5%��NaCl��Һ����ͼ�Ļ�ѧ���ʢݴ��ε��ᴿ��̽��ij���ε����ʡ����Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��
��ش�


(1)ָ����ͼ������a�����ƣ� �� д������ͼҩƷ��������ʵ��ʱ������Ӧ�Ļ�ѧ����ʽ��
(2)��ʵ��̨���ṩ��������ҩƷ������ΪС���鵽���ǵ� �����⣻
(3)������С����ɸ�ʵ�鲿�ֲ������̵�ʾ��ͼ��û��ָ���IJ�������ȷ�������ֱ���ÿ�������ȷ��1�֣�����10�֣�ʵ����Ϻ�С������8�֡����ҳ���ʧ�ֵIJ�����˵��ԭ�� �� ��
(4)������������(ҩƷ��ѡ)��Ҳ�������һ�ֳ��������ʵ������ȡ����ѧ����ʽΪ�� �����������ܺ������� (��һ�ֲ�����������)������װ�ɸ�������������ķ���װ�á�
��5������ͼ��ʾװ���ռ�����������Ӧ�� ���a����b�����˵��롣

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
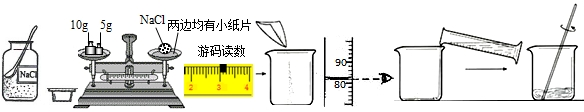
�����п���ѧʵ������������ĸ����⣺�ٴ����ᴿ����Ļ�ѧ���ʢ۶�����̼����ȡ���ռ�����������������ȡ���ռ������������Եķ������ɿ�����ǩȷ�����⣬С��ͬѧ��ǩ���ʦ��������������������ҩƷ��ʵ��̨ǰ��

��ش�
��ָ����ͼ������a�����ƣ� ��
����ʵ��̨���ṩ��������ҩƷ������ΪС��ͬѧ�鵽���ǵ� �����⣻
��������С��ͬѧ��ɸ�ʵ����Ҫ�������̵�ʾ��ͼ�������ֱ���ÿ�������ȷ��1�֣�����5�֣�ʵ����Ϻ�С��ͬѧ����3�֡����ҳ���ʧ�ֵIJ�����˵��ԭ��
�� ��

�ܽ�������������ҩƷ��ѡ����Ҳ�������һ�ֳ��������ʵ������ȡ����ѧ����ʽΪ�� �������� ����һ�ֲ����������ƣ�������װ�ɸ�������������ķ���װ�á�
��2����4�֣�������ʵ����ʱ��������һƿʧ���ǩ�İ�ɫ��ĩ��������̼���ƣ�Ҳ�������������ơ���ɫ��ĩ������������?����������벢���ʵ�����̽����
| �ҵIJ��� | �������� | ʵ������ | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com