


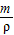 �������
�������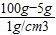 =95mL��
=95mL�� =10g��ˮ��������100g-10g=90g��
=10g��ˮ��������100g-10g=90g�� =95mL��
=95mL�� =10g��ˮ��������100g-10g=90g��
=10g��ˮ��������100g-10g=90g��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ�ɽ�����ݾ�����ѧ�������棩 ���ͣ�������
�ҹ���ȼú�����ȼú���鷢�����ҹ�һֱռ������λ��úȼ�ղ���������Ⱦ��絪�������������ȣ��������塢��������̬ϵͳΣ������������������������ǹ�ҵ������Ҷ���̿�չ��ͬʱ���������������о���������������һ���Ĺ�ҵӦ�á�������Һ��ͬʱ���յ������������̼���ܷ�ӦΪ��
2NO+2NO2+2SO2+O2+4CO��NH2��2��2CO2+4N2+2��NH4��2SO4
��ش��������⣺
��1�������������ѧ����ʽ��������Һ����NO��NO2��SO2����������__________��
��2��1000g������Һ��ȫ��Ӧ��������Ⱦ��õ��������132g������ʹ�õ�������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ���ȼú�����ȼú���鷢�����ҹ�һֱռ������λ��úȼ�ղ���������Ⱦ��絪�������������ȣ������塢��������̬ϵͳΣ������������������������ǹ�ҵ������Ҷ���̿�չ��ͬʱ���������������о���������������һ���Ĺ�ҵӦ�ã�������Һ��ͬʱ���յ���������������ܷ�ӦΪ��
O2+2NO+2NO2+2SO2+4CO��NH2��2�T4CO2+4N2+2��NH4��2SO4
��ش��������⣺
��1������������ѧ����ʽ��������Һ����NO��NO2��SO2������Ϊ������
��2��1000g������Һ��ȫ��Ӧ��������Ⱦ��õ������132g������ʹ��������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ���ȼú�����ȼú���鷢�����ҹ�һֱռ������λ��úȼ�ղ���������Ⱦ��絪�������������ȣ������塢��������̬ϵͳΣ������������������������ǹ�ҵ������Ҷ���̿�չ��ͬʱ���������������о���������������һ���Ĺ�ҵӦ�ã�������Һ��ͬʱ���յ���������������ܷ�ӦΪ��
O2+2NO+2NO2+2SO2+4CO��NH2��2═4CO2+4N2+2��NH4��2SO4
��ش��������⣺
��1������������ѧ����ʽ��������Һ����NO��NO2��SO2������Ϊ������
��2��1000g������Һ��ȫ��Ӧ��������Ⱦ��õ������132g������ʹ��������Һ���������������Ƕ��٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com