
���� ��1���ٸ������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ����������ӷ���ǰ������Ӧ�����ֽ��з�����
�ڸ���Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں���з�����
��2���ٸ��ݻ�������ɲ�ͬ������ɵ����ʽ��з�����
�ڸ��������к�������������ˮ��������������һ�������·�Ӧ���ɵ�����ˮ���з�����
��� �⣺��1�������ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ�����ʾ��������ӣ����������ӷ���ǰ������Ӧ�����֣���������������Һ�����ԭ������Һ�д��������ƶ��������ӡ����������ӣ���ѧʽΪ��Na+��OH-��
��Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں���������þ��þԪ����+2�۱�ʾΪ��$\stackrel{+2}{Mg}$O��
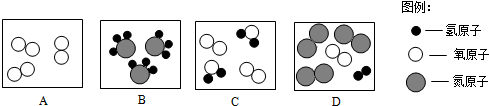
��2���ٻ�������ɲ�ͬ������ɵ����ʣ����ԴӺ�۽Ƕȿ���ͼ�б�ʾ��������CD��
�������к�������������ˮ��������������һ�������·�Ӧ���ɵ�����ˮ����ѧ����ʽΪ��3O2+4NH3$\frac{\underline{\;һ������\;}}{\;}$2N2+6H2O��
�ʴ�Ϊ����1����Na+��OH-��
��$\stackrel{+2}{Mg}$O��
��2����CD��
��3O2+4NH3$\frac{\underline{\;һ������\;}}{\;}$2N2+6H2O��
���� ������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
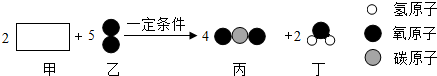
| A�� | ��Ӧǰ��ԭ�ӵ�����û�з����ı� | |
| B�� | �μӷ�Ӧ�ļס�����������13��20 | |
| C�� | ��Ԫ�صĻ��ϼ��ڷ�Ӧǰ�����˸ı� | |
| D�� | �÷�Ӧ����������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
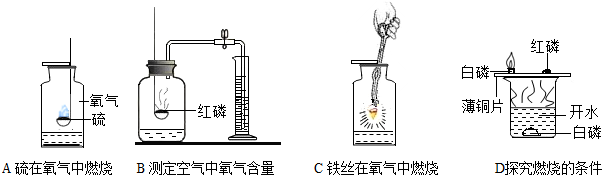
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �ڡ����-��-���š�֮�佨����ϵ����ѧϰ��ѧ��һ����Ҫ��˼ά��ʽ����ͼ��Ԫ�����ڱ��в���Ԫ�ص�ԭ�ӽṹʾ��ͼ��������ѧ֪ʶ�ش����е����⣮
�ڡ����-��-���š�֮�佨����ϵ����ѧϰ��ѧ��һ����Ҫ��˼ά��ʽ����ͼ��Ԫ�����ڱ��в���Ԫ�ص�ԭ�ӽṹʾ��ͼ��������ѧ֪ʶ�ش����е����⣮| ���� | ���� | ���� | |
| ��Է������� | 2 | 32 | 28 |
| ��״���£�22.4L��������� | 2g | 32g | Y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ú���ҩ��Ԥ����״���״� | |
| B�� | ʹ�����ƴ��߷���ȱ����ƶѪ | |
| C�� | ��ʳţ�̡�����Ʒ�ȸ��Ƶ�ʳ��Ԥ�������Ͳ� | |
| D�� | ʳ��ĵ�õȺ�п����Ʒ������ȱп����ķ���ͣ�͡��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
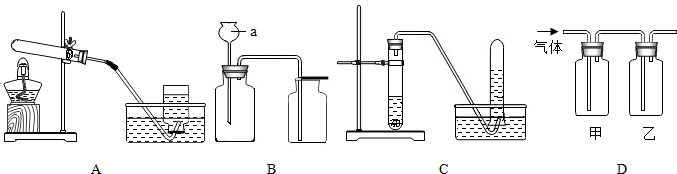
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
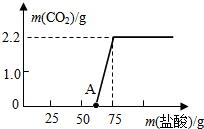 ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽����
ijУ�о���ѧϰС�������һ����Ȥ��ʵ��̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 33.3% | B�� | 66.7% | C�� | 80% | D�� | 74% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ѫ�쵰�����к�����Ԫ�أ�ȱ������������ƶѪ | |
| B�� | ���շ��������Ͽ��Լ��ٰ�ɫ��Ⱦ | |
| C�� | NaCl��NaNO2����ζ����������ʳ��ĵ�ζƷ | |
| D�� | �ڷ��͵������м���С�մ��������ͷ�Ŀڸкͳ�ȥ��ζ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com