
A��IΪ���л�ѧ�г��������ʣ���֪AΪ���������CΪ����ʯ����Ҫ�ɷ֣�DΪ��ɫ������G��I��Ϊֻ����һ�����ʵ���Һ����������ʾ���ʼ�������ת���Ĺ�ϵ(����������δ���)������ͼ��ʾ����ش��������⣺
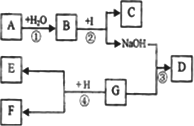
(1)д����ѧʽ��A________��D________��
(2)д��B��I��Ӧ�Ļ�ѧ����ʽ��________��
��Ӧ�١��ڡ��ۡ��������ڸ��ֽⷴӦ��Ϊ________(�����)��
(3)��֪HΪ���ۣ�����ҺG��ַ�Ӧ����ˣ�������E�м���ϡ���ᣬ����ɫ����ų���������E��һ������________����ҺF��һ�����еĽ�����������________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
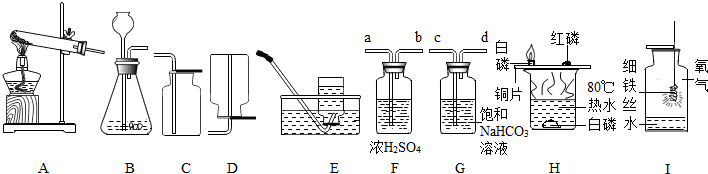
�����dz��г�����ʵ�飬������ѧ��ѧ֪ʶ���ش��й����⣺

��1��ʵ�����ø��������������װ��A����һ����������Ը��� ��
д����Ӧ�Ļ�ѧ����ʽ ������Cװ���ռ�������д����
���ķ��������������������ۣ� ��
��2��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ �����õķ���װ�ú�
�ռ�װ���� ��������ţ�
��3��ʵ�����ƵõĶ�����̼�����г���������HCl��ˮ������Ϊ���Ƶô����������
������̼���壬����װ�õĵ��ܰ�������������˳���� ������Ϳ��ţ�
A��a��b��c��d B��b��a��c��d
C��c��d��a��b D��d��c��b��a
��4������ʵ���ж��õ�ˮ��ͼH��I��ˮ�����÷ֱ���H�� I�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com