
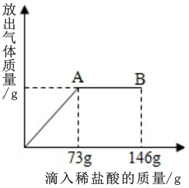
 ��һ�ձ���ʢ����Na2CO3��NaCl����ɵĹ�������25g���������μ�������������Ϊ10%��ϡ���ᣬ�ų���������������ϡ�����������ϵ��ͼ��ʾ����֪��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+CO2��+H2O�������������ͼ��ش��������⣺
��һ�ձ���ʢ����Na2CO3��NaCl����ɵĹ�������25g���������μ�������������Ϊ10%��ϡ���ᣬ�ų���������������ϡ�����������ϵ��ͼ��ʾ����֪��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+CO2��+H2O�������������ͼ��ش��������⣺���� ���ڸ��������ĵ���������������������������Ը���������HCl�������Ͷ�Ӧ�Ļ�ѧ����ʽ�����Ӧ��̼���Ƶ����������ɵ��Ȼ��Ƶ��������������������������
��1����������μӹ��̵õ���ͼ�������μ����ᵽ73gʱΪǡ����ȫ��Ӧ���ӵ�146gʱΪ�����������ʱpHС��7���з�����
��2��������������=��Һ�����������������������ݻ�ѧ����ʽ���м��㣻
��3�����ݲμӷ�Ӧ���Ȼ�������������ݻ�ѧ����ʽ����õ�̼���Ƶ�������Ȼ������Ȼ��Ƶ�����������
��� �⣺��1������ͼ���Կ��������μ�������73gʱҲ����A��ʱ����ʱ����ﵽ�������Ҳ����ǡ����ȫ��Ӧ����ʱ��ҺpH����7������ֻ���Ȼ��ƣ��������μ�����ʱ����������ʣ�࣬���Դ�ʱpHС��7������ΪHCl��NaCl��
��2����A��ʱ���ĵ�HCl������Ϊ73g��10%=7.3g��
������7.3gHClʱ��Ӧ��̼���Ƶ�����Ϊx�����ɵĶ�����̼������Ϊy
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 73 44
x 7.3g y
$\frac{106}{x}$=$\frac{73}{7.3g}$=$\frac{44}{y}$
x�T10.6g
y=4.4g
�������������̼��������4.4g��
��3�����Ի�������Ȼ��Ƶ�����Ϊ25g-10.6g�T14.4g
��������Ȼ��Ƶ���������Ϊ��$\frac{14.4g}{25g}$��100%�T57.6%��
�ʴ�Ϊ����1��=��NaCl������
��2��7.3��4.4g��
��3��57.6%��
���� ������Ҫ�����˻�ѧ����ʽ�ļ��㣬�ѶȲ���ע�����Ĺ淶�Ժ�ȷ�ԣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
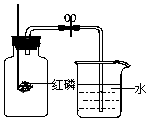 Ϊ�ⶨ�����������ĺ������ס��Ҷ���ͬѧ����ͼ��ʾ��װ�÷ֱ������ʵ��̽����
Ϊ�ⶨ�����������ĺ������ס��Ҷ���ͬѧ����ͼ��ʾ��װ�÷ֱ������ʵ��̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ά����A����Ԥ��ҹä֢ | |
| B�� | 1��ά����A1���ӱ�1��ά����A2���Ӷ�2��ԭ�� | |
| C�� | ά����A1��ά����A2���Ԫ����ͬ����ѧ����Ҳ��ͬ | |
| D�� | ά����A1����Ԫ�ر�ά����A2��Ԫ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na3PO4 | B�� | Na3PO4��Na4P2O7 | ||
| C�� | Na4P2O7����Na5P3O10 | D�� | Na3PO4��Na4P2O7����Na5P3O10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢڢ� | B�� | �ۢ٢ܢ� | C�� | �ۢܢ٢� | D�� | �٢ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
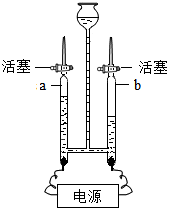 �������������벻��ˮ��
�������������벻��ˮ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com