
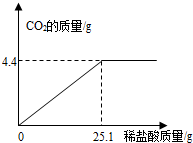
(7��) ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3 g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ����ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
��1����Ʒ��̼�����Ƶ�����������
��2��ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
ij��һ�����ʵ����ǵ����Ƹۼ�ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ��ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
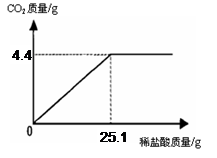
(7��) ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3 g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ����ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
��1����Ʒ��̼�����Ƶ�����������
��2��ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��������������п�ģ����Ի�ѧ�Ծ����ģ� ���ͣ�������
(7��) ij��һ�����ʵ����ǵ���ιۣ��ó���Ҫ��Ʒ֮һ��С�մ�̼�����ƣ����ι۽�����ͬѧ�Ǵ���һЩ�������������С�մ���Ʒ�����ⶨ����̼�����Ƶ������������������Ʒ��ֻ�����Ȼ���һ�����ʣ���ȡ��Ʒ9.3 g��μ���ϡ���ᣬ����CO2�����������μ�ϡ�����������ϵ����ͼ��ʾ�����������ðٷ�����ʾ��������С�����һλ���֣�
��1����Ʒ��̼�����Ƶ�����������
��2��ǡ����ȫ��Ӧʱ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com