ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������ˮ���˽�ˮ���й�֪ʶ�Ǻ��б�Ҫ�ģ�
��1����Ȼˮ�к����������ʣ�����ˮ��ͨ������Ȼˮ���г����� ��������������ʹ֮�ﵽ����ˮ������ʵ�������Ҫ�õ������̶���ߵ�ˮ������ �ķ�����
��2��ˮ������ÿ�춼���õ�����֮һ�����������ȹ����Ӳ�ȹ����ˮ�����������彡����ͨ�����ǿ��� �ⶨˮ�����ȣ����� ����Ӳˮ����ˮ�������г��� ����ʹӲˮ������
��3����Լ��ˮ��ÿ��������������·�ʽ��������ˮ���� ������ĸ��ţ�
A��������ˮ��ϴ��ˮ������������� B����ϵط�ˮϴ�·���
C������ϵط�ˮˢ���� D������ࡢ�ι�ķ�������ũ����֣�
���𰸡���������1�����ݾ�ˮ�ķ����Լ������̶ȵķ�����������н��
��2��������pH��ֽ�ⶨˮ�������Լ��÷���ˮ����Ӳˮ����ˮ�������г�����з���ʹӲˮ�������н��
��3������ֻҪ���Ͻ�Լ��ˮ���н��н��
����⣺��1����Ȼˮ�к����������ʣ�����ˮ��ͨ������Ȼˮ���г��������ˡ�������������ʹ֮�ﵽ����ˮ������ʵ�������Ҫ�õ������̶���ߵ�ˮ����������ķ�����������ˣ�����
��2����pH��ֽ�ⶨˮ�����ȣ��÷���ˮ����Ӳˮ����ˮ��������ĭ�������ˮ����ĭ�ٵ���Ӳˮ�������г�����з���ʹӲˮ���������pH��ֽ������ˮ����У�
��3��A��������ˮ��ϴ��ˮ���������������������һˮ���ã����Խ�Լ��ˮ��
B������ϵط�ˮϴ�·��������˷�ˮ��Դ��
C������ϵط�ˮˢ���������˷�ˮ��Դ��
D���ִ�ũ����ֲ�����ࡢ�ι�ȷ�ʽ�����Խ�Լ��ˮ��
��ѡ��AD��
�����������ؼ���Ҫ֪������ˮ�ľ������̣�֪�������г�����Լ��ˮ�ķ�����



 ��У����ϵ�д�
��У����ϵ�д�
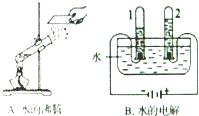 ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ������ˮ��Դ��
ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ������ˮ��Դ��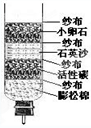 ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��С��ͬѧ������һ������ˮ������ͼ��ʾ��
ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��С��ͬѧ������һ������ˮ������ͼ��ʾ��
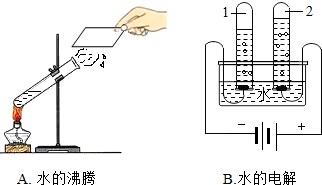 ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��
ˮ��������ԴȪ��Ϊ���������Ŀɳ�����չ������Ӧ���˽�ˮ���й�֪ʶ��