
 2nCH3CH2OH+2nCO2��[ע����C6H10O5��n����Է���������162n]��
2nCH3CH2OH+2nCO2��[ע����C6H10O5��n����Է���������162n]�� %��
%�� 2nCH3CH2OH+2nCO2��
2nCH3CH2OH+2nCO2��
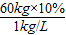 ×80mg/L=480mg��
×80mg/L=480mg�� %���Ƶ�����=
%���Ƶ�����=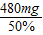 =960mg��
=960mg��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ�������Ϫ������ѧ ���ͣ������
�ƾ��ֳ�Ϊ�Ҵ���������������������������Ҳ��һ�����ȼ�ϡ��õ��������Ҵ��Ļ�ѧ��Ӧ����ʽ�ǣ�(C6H10O5)n�� nH2O  2nCH3CH2OH��2nCO2��
2nCH3CH2OH��2nCO2��
[ע��(C6H10O5)n����Է���������162n ]�����㣨�������1λС������
��1��CH3CH2OH����Է��������� ��
��2��100�K���������Ͽ����Ƶ��Ҵ��������Ƕ��٣�
��3���������Ƽݳ��ı�������ѪҺ���Ҵ���������80mg/L����������ѪҺռ�������ص�10%������ѪҺ���ܶ��ǣ�1kg/L������
�٣�һ������Ϊ60kg�ļ�ʻԱѪҺ�������Ҵ������������ܳ������٣�
�ڣ����н���൱����������Ϊ50%�İƵ������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011����б�ҵ��ѧ���ԣ�������Ϫ������ѧ ���ͣ������
�ƾ��ֳ�Ϊ�Ҵ���������������������������Ҳ��һ�����ȼ�ϡ��õ��������Ҵ��Ļ�ѧ��Ӧ����ʽ�ǣ�(C6H10O5)n��
nH2O  2nCH3CH2OH��2nCO2��
2nCH3CH2OH��2nCO2��
[ע��(C6H10O5)n����Է���������162n ]�����㣨�������1λС������
��1��CH3CH2OH����Է��������� ��
��2��100�K���������Ͽ����Ƶ��Ҵ��������Ƕ��٣�
��3���������Ƽݳ��ı�������ѪҺ���Ҵ���������80mg/L����������ѪҺռ�������ص�10%������ѪҺ���ܶ��ǣ�1kg/L������

�٣�һ������Ϊ60kg�ļ�ʻԱѪҺ�������Ҵ������������ܳ������٣�
�ڣ����н���൱����������Ϊ50%�İƵ������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����Ϫ ���ͣ��ʴ���
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�п����� ���ͣ�������
 2nCH3CH2OH+2nCO2��[ע��(C6H10O5)n����Է���������162n ]�����㣨�������1λС������
2nCH3CH2OH+2nCO2��[ע��(C6H10O5)n����Է���������162n ]�����㣨�������1λС�������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com