
 2”¢2007Äź4ŌĀ26ČÕ±±¾©°ĀŌĖ»š¾ę”°ĻéŌĘ”±ÕżŹ½¹«²¼£ØČēĶ¼£©£® ±±¾©°ĀŌĖ»į»š¾ęŹ¹ÓƵÄČ¼ĮĻĪŖ±ūĶ飬ÕāŹĒŅ»ÖÖ¼ŪøńµĶĮ®»·±£µÄ³£ÓĆČ¼ĮĻ£¬ĶźČ«Č¼ÉÕŗóֻɜ²ś¶žŃõ»ÆĢ¼ŗĶĖ®£¬¶Ō»·¾³Ć»ÓŠĪŪČ¾£®½öøł¾ŻÉĻŹöŠÅĻ¢£¬¶ŌČ¼ĮĻ±ūĶéµÄĶʶĻÕżČ·µÄŹĒ£Ø””””£©
2”¢2007Äź4ŌĀ26ČÕ±±¾©°ĀŌĖ»š¾ę”°ĻéŌĘ”±ÕżŹ½¹«²¼£ØČēĶ¼£©£® ±±¾©°ĀŌĖ»į»š¾ęŹ¹ÓƵÄČ¼ĮĻĪŖ±ūĶ飬ÕāŹĒŅ»ÖÖ¼ŪøńµĶĮ®»·±£µÄ³£ÓĆČ¼ĮĻ£¬ĶźČ«Č¼ÉÕŗóֻɜ²ś¶žŃõ»ÆĢ¼ŗĶĖ®£¬¶Ō»·¾³Ć»ÓŠĪŪČ¾£®½öøł¾ŻÉĻŹöŠÅĻ¢£¬¶ŌČ¼ĮĻ±ūĶéµÄĶʶĻÕżČ·µÄŹĒ£Ø””””£©

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2008?ĆÅĶ·¹µĒųŅ»Ä££©»ÆѧÖŖŹ¶ŌŚÉś²ś”¢Éś»īÖŠÓŠ¹ć·ŗµÄÓ¦ÓĆ£®
£Ø2008?ĆÅĶ·¹µĒųŅ»Ä££©»ÆѧÖŖŹ¶ŌŚÉś²ś”¢Éś»īÖŠÓŠ¹ć·ŗµÄÓ¦ÓĆ£®
| ||
| ||
 ĖłÓĆČ¼ĮĻ·ūŗĻ”°ĀĢÉ«°ĀŌĖ”±µÄŅŖĒó£¬ŌŅņŹĒ
ĖłÓĆČ¼ĮĻ·ūŗĻ”°ĀĢÉ«°ĀŌĖ”±µÄŅŖĒó£¬ŌŅņŹĒ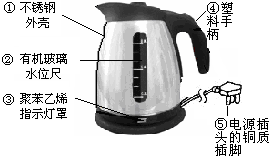
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| AӢC3H4 |
| BӢC3H6 |
| CӢC3H8O |
| DӢC3H8 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com