
(14·Ö)øł¾ŻĻĀĶ¼ĖłŹ¾,°“ŅŖĒó»Ų“šĪŹĢā:

£Ø1£©Ķ¼ÖŠŅĒĘ÷aµÄĆū³ĘŹĒ £¬Ķ¼FŹµŃéµÄÄæµÄŹĒ ”£
£Ø2£©ŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½ĪŖ £Ø2·Ö£©£»
ĖłŃ”ÓƵķ¢Éś×°ÖĆŹĒ £ØĢīŠņŗÅ£©”£
£Ø3£©ŹµŃéŹŅ¼ÓČČĀČĖį¼ŲŗĶ¶žŃõ»ÆĆĢÖĘŃõĘųŹ±£¬ŌŚøĆ·“Ó¦ÖŠÖŹĮæŗĶ»ÆѧŠŌÖŹ¶¼Ć»ÓŠøıäµÄĪļÖŹŹĒ £¬ŹÕ¼ÆŃõĘųĖłŃ”ÓƵÄ×°ÖĆŹĒ £ØĢīŠņŗÅ£©”£
£Ø4£©ŌŚ2011Äź5ŌĀæ¦×óĻŲ³õÉżøß»ÆѧŹµŃé²Ł×÷æ¼ŹŌÖŠ£¬Š”ŗģÓĆA”¢E×°ÖĆČĻÕęĶź³ÉĮĖĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢÖĘČ”ŃõĘųµÄŹµŃ飬Ēė¾Ż“ĖŹµŃé»Ų“šĻĀĮŠĪŹĢā”£
¢ŁĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”” ”””””””” ”””” ”££Ø2·Ö£©
¢ŚŌŚŹµŃé¹ż³ĢÖŠ£¬Š”¾üĶ¬Ń§ÓÉÓŚ¶Æ×÷Ģ«Āż£¬ŃõĘų»¹Ć»ÓŠŹÕ¼ÆĀś£¬£¬×¶ŠĪĘæÄŚµÄ·“Ó¦¾ĶŅŃ¾Ķ£Ö¹”£ČōĻė¼ÆĀśÕāĘæŃõĘų£¬ŌŚ²»²šŠ¶×°ÖƵÄĒ°ĢįĻĀ£¬ĒėÄć°ļĖūĻė³öæÉŠŠµÄ°ģ·Ø”£
·½·ØŹĒ ”££Ø1·Ö£©
¢ŪŠ”Ć÷Ķ¬Ń§ČĻĪŖ,ÕāĢ××°ÖƵÄȱµćŹĒ²»ÄÜæŲÖĘ·“Ó¦Ėꏱ·¢Éś”¢ĖꏱĶ£Ö¹,Ōģ³ÉĮĖŅ©Ę·µÄĄĖ·Ń”£ĪŖĮĖ½ā¾öŠ”Ć÷Ģį³öµÄĪŹĢā,ĒėÄć¶ŌŌŹµŃé×°ÖĆ¼ÓŅŌøĽų,»ņÉč¼ĘŠĀµÄ¼ņŅ׏µŃé×°ÖĆ,ŅŌ±ćÓŠŠ§µŲæŲÖĘĖ«ŃõĖ®µÄ·Ö½ā·“Ó¦”£
ÓŃĒéĢįŹ¾:ÄćæÉ“ÓĻĀĮŠÓĆʷ֊єȔ,Ņ²æÉ×ŌŠŠŃ”ÓĆĘäĖüŅĒĘ÷”£

ĒėÄćŌŚĻĀ±ķÖŠÖĮÉŁŠ“³öĮ½ÖÖ·½°ø£ŗ£Ø4·Ö£©
|
·½°øŅ» |
·½°ø¶ž |
|
|
|
£Ø1£©¼ÆĘųĘæ£Ø1·Ö£© ¼ģ²é×°ÖƵÄĘųĆÜŠŌ£Ø1·Ö£© £Ø2£©CaCO3 + 2HCl = CaCl2 + H2O + CO2”ü£»£Ø2·Ö£© A£Ø1·Ö£©£Ø3£©¶žŃõ»ÆĆĢ£Ø»ņMnO2£©£Ø1·Ö£© D»ņE£Ø1·Ö£©
£Ø4£©¢Ł2H2O2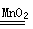 2H2O+O2”ü£Ø2·Ö£©
2H2O+O2”ü£Ø2·Ö£©
¢Ś ŌŁ¼ÓČėŹŹĮæµÄĖ«ŃõĖ®Ź¹·“Ó¦¼ĢŠų½ųŠŠ»ņ“Ó³¤¾±Ā©¶·Ļņ׶ŠĪĘæÄŚ¼ÓĖ®£¬½«×¶ŠĪĘæÄŚµÄŃõĘųŃ¹Čė¼ÆĘųĘæÖŠ£Ø1·Ö,ĘäĖū“š°øŗĻĄķ¾łøų·Ö£©
¢Ū a½«³¤¾±Ā©¶·øÄÓĆ·ÖŅŗĀ©¶· b »ņ½«³¤¾±Ā©¶·øÄÓĆ×¢ÉäĘ÷ c »ņ½«³¤¾±Ā©¶·øÄÓĆ½ŗĶ·µĪ¹Ü d»ņŃ”ÓĆŹ¢×°¶žŃõ»ÆĆĢµÄŠ”²¼“ü,½«Į¬ŌŚŠ”“ü×ÓÉĻµÄĢśĖæ“©¹żĻšĘ¤Čū²¢ÉĻĻĀ³é¶Æ e »ņ·ĀĘōĘÕ·¢ÉśĘ÷ŌĄķµÄ¼ņŅ׏µŃé×°ÖĆ”£””(Ć抓³öŅ»ÖÖŗĻĄķµÄ·½°øøų2·Ö,×ī¶ąµĆ4·Ö)
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗ£Ø1£©Źģ¼Ē³£ÓĆŅĒĘ÷£¬ĮĖ½ā³£ÓƵÄŅĒĘ÷Ćū³Ę”¢ÓĆ·Ø”¢Ń”Č”ŗĶĮ¬½Ó·½·ØµČ£¬Ķ¼ÖŠŅĒĘ÷aµÄĆū³ĘŹĒ¼ÆĘųĘ棻Ķ¼FŹµŃéµÄÄæµÄŹĒ¼ģ²é×°ÖƵÄĘųĆÜŠŌ£»
£Ø2£©ŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½ĪŖCaCO3 + 2HCl = CaCl2 + H2O + CO2”ü£»ŹōÓŚµäŠĶµÄ¹ĢŅŗĢå·“Ó¦ĒŅ²»ŠčŅŖ¼ÓČȵĥąŠĶ£¬øł¾Ż·“Ó¦ĪļµÄדĢ¬ŗĶ·“Ó¦Ģõ¼žæÉÖŖ£¬ĖłŃ”ÓƵķ¢Éś×°ÖĆŹĒA£»
£Ø3£©ŹµŃéŹŅ¼ÓČČĀČĖį¼ŲŗĶ¶žŃõ»ÆĆĢÖĘŃõĘųŹ±£¬ŌŚøĆ·“Ó¦ÖŠÖŹĮæŗĶ»ÆѧŠŌÖŹ¶¼Ć»ÓŠøıäµÄĪļÖŹŹĒ“߻ƼĮ¶žŃõ»ÆĆĢ£»øł¾ŻŃõĘųµÄĪļĄķŠŌÖŹ£ŗĆܶȱČæÕĘų“ó£¬ĒŅ²»Ņ×ČÜÓŚĖ®£¬¹ŹŹÕ¼ÆŃõĘųĖłŃ”ÓƵÄ×°ÖĆŹĒD»ņE£»
£Ø4£©¢ŁĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢ·¢Éś·“Ӧɜ³ÉĖ®ŗĶŃõĘų£¬ĘäÖŠ¶žŃõ»ÆĆĢ×öøĆ·“Ó¦µÄ“߻ƼĮ£¬¹ŹøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ2H2O2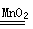 2H2O+O2”ü£»
2H2O+O2”ü£»
¢ŚŅņĪŖ¶žŃõ»ÆĆĢŌŚ»Æѧ·“Ó¦Ē°ŗó»ÆѧŠŌÖŹ²»±ä£¬ĖłŅŌæÉŌŁ¼ÓČėŹŹĮæµÄĖ«ŃõĖ®Ź¹·“Ó¦¼ĢŠų½ųŠŠ£»ÓÖŅņĪŖ·“Ó¦½įŹųŗó£¬×¶ŠĪĘæÄŚČŌȻӊŃõĘų²ŠĮō£¬¹ŹæÉ“Ó·ÖŅŗĀ©¶·Ļņ׶ŠĪĘæÄŚ¼ÓĖ®£¬½«×¶ŠĪĘæÄŚµÄŃõĘųŃ¹Čė¼ÆĘųĘæÖŠ£»
¢ŪĪŖĮĖæŲÖĘ·“Ó¦ĖŁĀŹæÉŅŌ½«³¤¾±Ā©¶·øÄÓĆ·ÖŅŗĀ©¶·»ņ×¢ÉäĘ÷£¬ÕāŃłæÉæŲÖĘ¹żŃõ»ÆĒāµÄµĪ¼ÓĖŁ¶Č£¬¼“æÉæŲÖĘ·“Ó¦µÄĖŁĀŹ£»»ņÕßŃ”ÓĆŹ¢×°¶žŃõ»ÆĆĢµÄŠ”²¼“ü£¬½«Į¬ŌŚŠ”“ü×ÓÉĻµÄĢśĖæ“©¹żĻšĘ¤Čū²¢ÉĻĻĀ³é¶Æ£¬Ņ²ÄÜĘšµ½æŲÖĘ·“Ó¦µÄ·¢ÉśÓėĶ£Ö¹”£
æ¼µć£ŗ³£ÓĆĘųĢåµÄ·¢Éś×°ÖĆŗĶŹÕ¼Æ×°ÖĆÓėєȔ·½·Ø£¬³£ÓĆŅĒĘ÷µÄĆū³ĘŗĶŃ”ÓĆ£¬ŹµŃéŹŅÖĘČ”ŃõĘųµÄ·“Ó¦ŌĄķ£¬“߻ƼĮµÄĢŲµćÓė“ß»Æ×÷ÓĆ£¬ŹéŠ“»Æѧ·½³ĢŹ½£¬
µćĘĄ£ŗ±¾Ģāæ¼²éµÄÖŖŹ¶µćŗÜČ«Ćę£¬¼ČÓŠŃ”ŌńÖĘČ”×°ÖĆÓÖÓŠŃ”ŌńŹÕ¼Æ×°ÖĆ£¬»¹ÓŠ¶ŌĘųĢåÖĘČ”×°ÖƵÄøĽų£¬ÓÅ»ÆĮĖŅĒĘ÷µÄ×éŗĻ£¬æŖĶŲĮĖѧɜµÄĖ¼Ī¬”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø14·Ö£©ŹµŃéŹŅÖĘČ”ĘųĢåµÄ³£ÓĆ×°ÖĆČēĻĀĶ¼ĖłŹ¾£¬øł¾ŻĖłŃ§µÄÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā”£
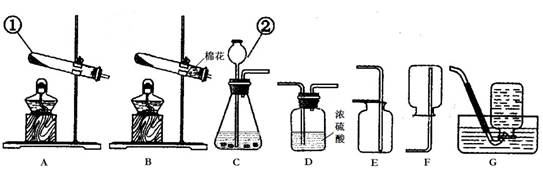
£Ø1£©Š“³ö×°ÖĆÖŠ±źŗÅŅĒĘ÷µÄĆū³Ę£ŗ¢Ł £»¢Ś ”£
£Ø2£©ŹµŃéŹŅÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘų£¬æÉŃ”ÓƵķ¢Éś×°ÖĆŹĒ £ØĢī×ÖÄø£©”£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©Š“³öŹµŃéŹŅÓĆ“óĄķŹÆŗĶĻ”ŃĪĖįÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½£ŗ ²¢¾Ż“ĖŃ”ŌńÉĻĶ¼ÖŠ £ØĢī×ÖÄø£©×é×°Ņ»Ģ×ÖĘČ”øÉŌļ¶žŃõ»ÆĢ¼µÄ×°ÖĆ”£
£Ø4£©Š”Ć÷½«ŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼ŗóµÄ·ĻŅŗ¾²ÖĆ£¬Č”ÉĻ²ćĒåŅŗ50g£¬ĻņĘäÖŠÖšµĪ¼ÓČėÖŹĮæ·ÖŹżĪŖ26.5%µÄĢ¼ĖįÄĘČÜŅŗ”£Ėżøł¾ŻŹµŃé²āµĆµÄŹż¾Ż»ę³öĻĀĶ¼£¬Ęä֊ׯ×ų±źmŹĒŹµŃéµĆµ½µÄ³Įµķ»ņĘųĢåµÄÖŹĮ棬ŗį×ų±ź±ķŹ¾µÄŹĒ¼ÓČėĢ¼ĖįÄĘČÜŅŗµÄÖŹĮ攣ŹŌ¼ĘĖć£ŗ
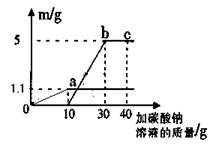
¢Ł50g·ĻŅŗÖŠŗ¬ĀČ»ÆøʵÄÖŹĮ棻
¢Śbµć±ķŹ¾µÄČÜŅŗÖŠĀČ»ÆÄʵÄÖŹĮæ·ÖŹż”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ¹ć¶«Ź”ČÄĘ½ĻŲ¾ÅÄź¼¶¼¶½Ģѧ֏Įæµ÷ŃŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĪŹ“šĢā
£Ø14·Ö£©ĻĀĶ¼AÓėBŹĒŹµŃéŹŅ³£ÓĆĄ“ÖĘČ”ĘųĢåµÄ×°ÖĆ£¬øł¾ŻĖłŃ§ÖŖŹ¶»Ų“šŅŌĻĀĪŹĢā£ŗ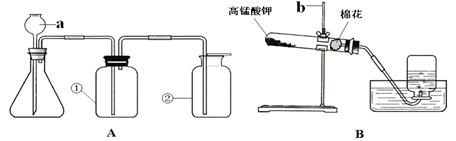
£Ø1£©Š“³öĶ¼ÖŠ±źŗÅŅĒĘ÷µÄĆū³Ę£ŗa £¬b ”£
£Ø2£©×°ÖĆB±ķŹ¾ÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘų£¬ĘäÖŠ»¹Č±ÉŁµÄŅĒĘ÷ŹĒ £ØĢīĆū³Ę£©£¬Ķ¼ÖŠĆŽ»ØµÄ×÷ÓĆŹĒ £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
”£”°A”±»ņ”°B”±¾łæÉÖĘČ”ŃõĘų£¬”°A”±Óė”°B”±±Č½Ļ£¬”°A”±¾ßÓŠµÄÓŵćŹĒ ”£
£Ø3£©ČōĻėŌŚŹµŃéŹŅÖĘČ””¢ŹÕ¼Æ”¢¼ģŃ鶞Ńõ»ÆĢ¼²¢ŃéÖ¤ĘäŠŌÖŹ£¬Ó¦Ń”ŌńA×°ÖĆ£¬ŌŚaÖŠĢķ¼ÓµÄŅ©Ę·ĪŖ £¬ŌŚ×¶ŠĪĘæÖŠ·ÅÖƵÄŅ©Ę·ĪŖ £¬“Ė·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ŅŖŃéÖ¤¶žŃõ»ÆĢ¼£¬ŌŚ¢ŁÖŠ·Å³ĪĒåŹÆ»ŅĖ®£¬ĻÖĻóĪŖ ”£
£Ø5£©ŅŖŃéÖ¤¶žŃõ»ÆĢ¼ÓėĖ®·“Ó¦£¬ŌŚ¢ŁÖŠ·Å×ĻÉ«ŹÆČļŹŌŅŗ£¬ĻÖĻóĪŖ £»ÓĆ“Ė·½·ØŃéÖ¤¶žŃõ»ÆĢ¼ÓėĖ®·“Ó¦µÄ²»Ē”µ±ĄķÓÉŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2013½ģĮÉÄžŹ”½ØĘ½ĻŲ¾ÅÄź¼¶ÉĻŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢ½¾æĢā
(14·Ö)øł¾ŻĻĀĶ¼ĖłŹ¾,°“ŅŖĒó»Ų“šĪŹĢā:
£Ø1£©Ķ¼ÖŠŅĒĘ÷aµÄĆū³ĘŹĒ £¬Ķ¼FŹµŃéµÄÄæµÄŹĒ ”£
£Ø2£©ŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼µÄ»Æѧ·½³ĢŹ½ĪŖ £Ø2·Ö£©£»
ĖłŃ”ÓƵķ¢Éś×°ÖĆŹĒ £ØĢīŠņŗÅ£©”£
£Ø3£©ŹµŃéŹŅ¼ÓČČĀČĖį¼ŲŗĶ¶žŃõ»ÆĆĢÖĘŃõĘųŹ±£¬ŌŚøĆ·“Ó¦ÖŠÖŹĮæŗĶ»ÆѧŠŌÖŹ¶¼Ć»ÓŠøıäµÄĪļÖŹŹĒ £¬ŹÕ¼ÆŃõĘųĖłŃ”ÓƵÄ×°ÖĆŹĒ £ØĢīŠņŗÅ£©”£
£Ø4£©ŌŚ2011Äź5ŌĀæ¦×óĻŲ³õÉżøß»ÆѧŹµŃé²Ł×÷æ¼ŹŌÖŠ£¬Š”ŗģÓĆA”¢E×°ÖĆČĻÕęĶź³ÉĮĖĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢÖĘČ”ŃõĘųµÄŹµŃ飬Ēė¾Ż“ĖŹµŃé»Ų“šĻĀĮŠĪŹĢā”£
¢ŁĖ«ŃõĖ®ŗĶ¶žŃõ»ÆĆĢ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”” ”””””””” ”””” ”££Ø2·Ö£©
¢ŚŌŚŹµŃé¹ż³ĢÖŠ£¬Š”¾üĶ¬Ń§ÓÉÓŚ¶Æ×÷Ģ«Āż£¬ŃõĘų»¹Ć»ÓŠŹÕ¼ÆĀś£¬£¬×¶ŠĪĘæÄŚµÄ·“Ó¦¾ĶŅŃ¾Ķ£Ö¹”£ČōĻė¼ÆĀśÕāĘæŃõĘų£¬ŌŚ²»²šŠ¶×°ÖƵÄĒ°ĢįĻĀ£¬ĒėÄć°ļĖūĻė³öæÉŠŠµÄ°ģ·Ø”£
·½·ØŹĒ ”££Ø1·Ö£©
¢ŪŠ”Ć÷Ķ¬Ń§ČĻĪŖ,ÕāĢ××°ÖƵÄȱµćŹĒ²»ÄÜæŲÖĘ·“Ó¦Ėꏱ·¢Éś”¢ĖꏱĶ£Ö¹,Ōģ³ÉĮĖŅ©Ę·µÄĄĖ·Ń”£ĪŖĮĖ½ā¾öŠ”Ć÷Ģį³öµÄĪŹĢā,ĒėÄć¶ŌŌŹµŃé×°ÖĆ¼ÓŅŌøĽų,»ņÉč¼ĘŠĀµÄ¼ņŅ׏µŃé×°ÖĆ,ŅŌ±ćÓŠŠ§µŲæŲÖĘĖ«ŃõĖ®µÄ·Ö½ā·“Ó¦”£
ÓŃĒéĢįŹ¾:ÄćæÉ“ÓĻĀĮŠÓĆʷ֊єȔ,Ņ²æÉ×ŌŠŠŃ”ÓĆĘäĖüŅĒĘ÷”£
ĒėÄćŌŚĻĀ±ķÖŠÖĮÉŁŠ“³öĮ½ÖÖ·½°ø£ŗ£Ø4·Ö£©
| ·½°øŅ» | ·½°ø¶ž |
| | |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”ČÄĘ½ĻŲ¾ÅÄź¼¶¼¶½Ģѧ֏Įæµ÷ŃŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ¼ņ“šĢā
£Ø14·Ö£©ĻĀĶ¼AÓėBŹĒŹµŃéŹŅ³£ÓĆĄ“ÖĘČ”ĘųĢåµÄ×°ÖĆ£¬øł¾ŻĖłŃ§ÖŖŹ¶»Ų“šŅŌĻĀĪŹĢā£ŗ
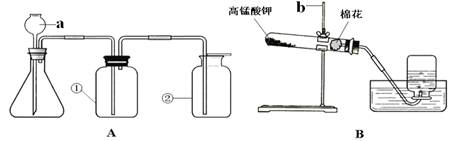
£Ø1£©Š“³öĶ¼ÖŠ±źŗÅŅĒĘ÷µÄĆū³Ę£ŗa £¬b ”£
£Ø2£©×°ÖĆB±ķŹ¾ÓĆøßĆĢĖį¼ŲÖĘČ”ŃõĘų£¬ĘäÖŠ»¹Č±ÉŁµÄŅĒĘ÷ŹĒ £ØĢīĆū³Ę£©£¬Ķ¼ÖŠĆŽ»ØµÄ×÷ÓĆŹĒ £¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
”£”°A”±»ņ”°B”±¾łæÉÖĘČ”ŃõĘų£¬”°A”±Óė”°B”±±Č½Ļ£¬”°A”±¾ßÓŠµÄÓŵćŹĒ ”£
£Ø3£©ČōĻėŌŚŹµŃéŹŅÖĘČ””¢ŹÕ¼Æ”¢¼ģŃ鶞Ńõ»ÆĢ¼²¢ŃéÖ¤ĘäŠŌÖŹ£¬Ó¦Ń”ŌńA×°ÖĆ£¬ŌŚaÖŠĢķ¼ÓµÄŅ©Ę·ĪŖ £¬ŌŚ×¶ŠĪĘæÖŠ·ÅÖƵÄŅ©Ę·ĪŖ £¬“Ė·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©ŅŖŃéÖ¤¶žŃõ»ÆĢ¼£¬ŌŚ¢ŁÖŠ·Å³ĪĒåŹÆ»ŅĖ®£¬ĻÖĻóĪŖ ”£
£Ø5£©ŅŖŃéÖ¤¶žŃõ»ÆĢ¼ÓėĖ®·“Ó¦£¬ŌŚ¢ŁÖŠ·Å×ĻÉ«ŹÆČļŹŌŅŗ£¬ĻÖĻóĪŖ £»ÓĆ“Ė·½·ØŃéÖ¤¶žŃõ»ÆĢ¼ÓėĖ®·“Ó¦µÄ²»Ē”µ±ĄķÓÉŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com